 About Authors: Ashwini K. Reddy, Swetha, Gauthami, Srivally, Masoom - M.Pharm, Helen Sheeba - Assistant Professor. Malla Reddy Institute of Pharmaceutical Sciences, Hyderabad.
About Authors: Ashwini K. Reddy, Swetha, Gauthami, Srivally, Masoom - M.Pharm, Helen Sheeba - Assistant Professor. Malla Reddy Institute of Pharmaceutical Sciences, Hyderabad.
Reference ID: PHARMATUTOR-ART-1076
HIGH - THROUGHPUT screening (HTS) is an approach to drug discovery that has gained widespread popularity over the last three or four years. HTS is the process of assaying a large number of potential effectors of biological activity against targets (a biological event). The methods of HTS are applied to the screening of combinatorial chemistry, genomics, protein, and peptide libraries. The goal of HTS is to accelerate drug discovery by screening large libraries often composed of hundreds of thousands of compounds (drug candidates) at a rate that may exceed 20,000 compounds per week. This paper will focus on assay adaptations, robotic equipment, and implementation strategies that allow HTS programs to be successful. Ultrahigh throughput screening (UHTS) issues (testing of 100,000 compounds / day) will also be discussed.
Due to the need to process thousands of assays per day, HTS has revolved around the combined world of multiple-well microplates and robotic processing. For a number of years, HTS assays have been run in the standard 96 - well microplate (working volume of up to 250 μL). The current
goal of most companies is to move beyond this format to higher - density, lower - volume formats (e.g., 384 - and 1536 - well microplates). There are two primary advantages of these formats: increased throughput and lower volume, which translates into lower cost. At screening rates of 500,000 compounds / week, a cost of USD 1 per well is difficult for any company’s budget to support on a weekly basis.
The reduction of cost, rather than increase in throughput, is the primary driving force within most HTS groups to move to higher- density, lower-volume microplates. HTS is only one step in the early drug discovery process. Other steps include compound library construction, secondary screening, and compound library optimization through medicinal chemistry. Many companies that have developed automated HTS systems find their drug discovery rate-limiting step is often assay/target development or medicinal chemistry. The time frame for these steps often exceeds the 2–3 months it will take to screen a compound library. However, the increased throughput is obviously a welcome benefit of high-density plate formats. In previous years, the primary hurdles for moving to a more dense microplate format (i.e., 384 - well microplate) have been a combination of adapting assays to lower volumes, reliable 384 - well pipetting equipment, and plate readers for 384 - well plates. The past year has seen the development of reliable 384 - well pipettors and readers that are 384 - well capable.
S u r p r i s i n g l y, despite the presence of 384-well assays, the majority of screening groups are still performing the bulk of their screening in 96-well plates. Some of the hurdles now preventing the fast migration of assays to 384-well format include assay sensitivity, surface tension and therefore mixing issues in 384-well plates, and format incompatibilities between 384-well screens and library compounds stored in 96-well microplates.
Assay design companies, rather than pharmaceutical and biotechnology companies, have been the fastest to explore and validate 384-well assays (e.g., the TR717 ). However, some pharmaceutical companies with advanced assay development groups have demonstrated impressive 384-well and 1536-well assay data.
Assay design
Generally speaking, first-pass HTS assays (the primary screen) are less quantitative than traditional biological assays. Often, compounds are only tested in duplicate (an increasing number of companies are using singlets), and usually at one concentration (most often in the 1–10 μM range for combinatorial chemistry libraries). If a “hit” (positive) is discovered, more accurate secondary assays are used for follow-up, quantification, and I C5 0 calculation. The advantages of using betterquality assays in primary screens will be discussed later in this section. HTS uses standard assay types known to most biological and biochemical scientists (e.g., ELISA, proliferation / cytotoxicity assays, reporter assays, and binding assays). However, adaptations of these assays have emerged to facilitate throughput and relieve robotic complexity. Screeners define assays as either heterogeneous or homogeneous. Heterogeneous assays require steps that go beyond simple fluid additions, incubations, and reading (e.g., filtration, centrifugation, and plate washing steps). Homogeneous assays require only additions and incubations followed by reading (e.g, luciferase assay).
True homogeneous assays involve simply mixing all the assay ingredients in one step, incubating, and reading the assay plate (Figure 2, LucScreen on Allegro). However, most assays still referred to as homogeneous require multiple additions and incubations at different times within a procedure followed by a final read step. Despite the advantages of homogeneous assays, the HTS community continues to use heterogeneous assays (e.g., ELISA) in a good percentage of screens. Primary drivers for the use of homogeneous assays are their speed (very few steps, which aids throughput) and simplicity. Asimple assay will reduce the robotic complexity requirements for automation. Past experience has shown that the automation of such tasks as microplate filtration, centrifugation,and washing has led to lower assay sensitivity (compared to manual processing) and costly robotics.
Within the last year, new robotics, such as the Allegro, and second-generation microplate devices (e.g., plate washers), now provide more robust approaches to processing heterogeneous assays both quickly and with a good assay signal. Additionally, in past years, robotic processing of heterogeneous assays was far slower than that of homogeneous assays. Assembly- line-style robotics (discussed below) can process heterogeneous assays at the same rate as homogeneous assays. Therefore, given the correct equipment environment, the advantages of moving to homogeneous assays are now fewer.
For example, Tropix has performed a UHTS heterogeneous kinase inhibitor screen that processed one 96-well microplate/min for 24 hr. A s mentioned above, the advantage of traditional heterogeneous assays is that their sensitivity and signal-to-noise ratios still exceed most homogeneous assay equivalents. The higher-quality assay data will result in fewer false negatives and false positives, reducing the potential miss of a hit and the expense of screening compounds that do not need to be rescreened. The desire for more sensitive nonradioactive screening assays is furthering the use of chemiluminescence and fluorescent assay techniques. Chemiluminescent assays provide the most sensitive assay detection method, offering the strongest combination of dynamic range and sensitivity . 2 An example of potential future assay technologies is shown in Figure .1 in an assay using chemiluminescent detection, a 1-μL total assay volume, and CCD c a m e r aimaging (replacing a plate reader). Similar miniaturization efforts are being used for fluorescent technologies at such companies as Evotec ( Hamburg, Germany) and Aurora (San Diego, CA).
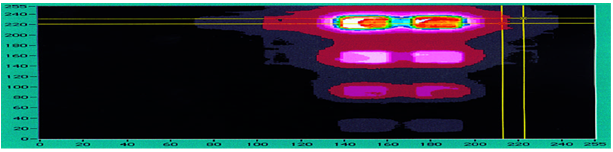
Charge coupled device (CCD) - acquired image of an SRC kinase chemiluminescent assay (alkaline phos phatase induced) in a 1 μLmicroarray system
HTS robotics
The marriage of robotics and HTS has been important to achieve the desired screening rates, as well as relieving scientific staff from tedious work. However, until the last year or so, one could argue that robotics for screening has been more of a research endeavor that a true implementation of stable technology.
Problems associated with screening robotics have included long design and implementation time, long manual to automated method transfer time, nonstable robotic operation, and limited error recovery abilities. These problems can be attributed to robot integration architectures, poor software design, and robot–workstation compatibility issues (e.g., microplate readers and liquid h a n d l e r s3, 4). Tr a d i t i o n a l l y, these integrated robot architectures have involved multiple layered computers, different operating systems, a single central robot servicing all peripheral devices, and the necessity of complex scheduling software to coordinate all of the above3–5 (Figure 2).
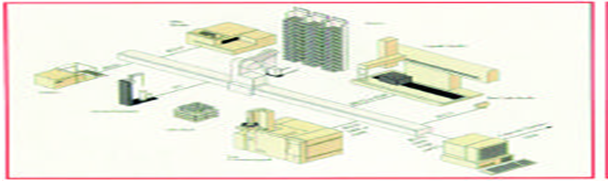
Figure 2 Robot-centric microplate processing system that uses an ORCA arm for plate transportation
These robot - centric HTS systems have a central robot with a gripper that can pick and place microplates around a platform (Figure 2). T h e y typically process between 40 and 100 microplates in a single run (the duration of the run depends on the assay type). The screener loads the robotic platform with microplates and reagents at the beginning of the experiment and the assay is then processed unattended. Robotic HTS systems often possess humidified CO2 incubators and are enclosed for tissue culture work. A new and improved industrial approach to HTS robotics is the Allegro technology . Similar to assembly-line manufacturing, microplates are passed down a line in serial fashion to consecutive processing modules. Each module has its own simple pick and place robotic arm (to pass plates to the next module) and microplate processing device. Therefore, at each module, one step of the assay is completed. This arrangement, coupled with Windows NT™ (Microsoft, Redmond,WA) and an Ethernet TCP/IP link between modules, provides a much simpler and more stable platform than robot-centric HTS systems.
Additionally, there is no complex scheduling software required and throughput rates are much higher. The slowest module will determine the plate throughput, and this is often a read step, usually in the range of 1–2 min in duration. Therefore, for many assays, Allegro can process microplates at a rate of one every 1–2 min (throughputs of 750–1000 microplates per day are possible). The importance of high-throughput robotics not only relates to being able to process very large libraries, but also enables fast turnaround of screening data in a very short time (regardless of library size). This increases the speed of the overall drug discovery process, a crucial issue for all drug discovery companies.
Screening Expense and Outsourcing Screening
Very few companies wish to screen 100,000 compounds per day in-house. The reasons for this include drug discovery process bottlenecks, equipment/robotic requirements, infrastructure investment, and limited need to invest in changing technologies. Some specific costs related to screening are assay reagent costs (reagents, cell culture expenses, etc.), microplate costs, pipet tip box costs, screening employee costs, data handling/ analysis time, database costs, robot purchase costs, and laboratory space costs. Due to the combined difficulties of the above, a growing number of contract screening companies are e m e rging (such as Tropix; PanLabs, Seattle, WA; and Evotec). The services of these companies usually include assay development and screening, data analysis, and other library support needs for HTS. Since many of these companies no longer require royalty payments if a drug is discovered, their use is becoming more popular. Contract screening companies are also being used for their ability to provide assay data with very fast turnaround times. They achieve this by running 24-hr shifts and using HTS robotic technologies.
Additionally, some companies choose to outsource primary screening, since they are finding the need to move some of their screening personnel to growing secondary screening programs. This keeps the higher-value, more proprietary secondary screening in-house, and enables the maintenance of a high rate of hit generation derived from outsourced primary screening.
Summary
The HTS field continues to be dynamic and very competitive. However, there is also a good deal of information sharing, even between competing companies. The somewhat open technology transfer meetings and publications have greatly accelerated the development of the industry. It is an industry that has been significantly driven by implementing technologies from vendor companies rather than through developments occurring within drug research companies. The future will certainly hold change as the industry strives to reach such high-density formats as the 1536 - well plate, and such technologies as microassay systems using nanoscale capillaries. 7, 8 H o w e v e r, for the moment, these technologies are still a few years away from routine in-house screening.
References
1. Comley JCW, Reeves T, Robinson P. A 1536 colorimetric SPAP reporter assay: comparison with 96- and 384-well formats. J Biomolec Screen 1998; 3(3):217.
2. Hengen PN. Chemiluminescent detection methods. Trends Biol Sci Aug 1997.
3. Hamilton S, Armstrong JW, Gerren RA, Janssen AM, Petersen J, Stanton RA. An overview of automated biotechnology screening. Lab Automat Robot 1996; 8(5):287–94.
4. Armstrong JW. HTS and robotics implementation: a critique of robotics equipment and strategies. Thousand Oaks, CA: HTS Consulting Ltd., 1997: 19–33.
5. Armstrong JW, Gerren RA, Hamilton SD. A review of automation options to support plate preparation, cherry picking and homogeneous assays. J Biomolec Screen 1999; 4:1.
6. Alderman E, Elands J. A novel approach to ultra-high throughput screening. Genet Eng News Jan 1998; 18(2).
7. Rose D, Lenmo T. Challenges in implementing high-density formats for high throughput screening. Lab Automat News Sept 1997; 2:4.
8. Knapp M. Microfluidic systems for high-throughput screening. Proceedings 4th Annual Conference and Exhibition:Society for Biomolecular Screening, San Diego, CA, 1998.
Figure 1 Charge coupled device (CCD)-acquired image of an SRC kinase chemiluminescent assay (alkaline phos - phatase induced) in a 1-μLmicroarray system (data courtesy of Tropix).
Figure 2 Robot-centric microplate processing system that uses an ORCA arm for plate transportation (diagram cour - tesy of Sagian, Indianapolis, IN).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org









