ABOUT AUTHORS
BHANDARI SALONI *, KISHNANI KHUSHBOO, RATHORE KAMAL SINGH
BN College of Pharmacy, Udaipur-Raj. 313001
salonibhandari1996.sb@gmail.com
ABSTRACT
Liposomes are concentric bilayer vesicles, which were first developed by Bangham and his colleagues in 1961. They are highly efficient having high drug entrapment capacity. Due to their size, hydrophobic and lipophilic character they are most widely used vehicles for drug delivery. The main aim of this drug delivery system is to target the drug directly to the site of action in order to prolong and enhance the drug effect. Liposomes are biocompatible and stable and are able to entrap both hydrophilic and lipophilic drug within its compartment. The size range varies from 0.05-5.0µ in diameter. Various convectional techniques used for liposomal preparation and size reduction are mechanical dispersion methods, solvent dispersion methods and detergent removal method. Due to difference in method of preparation and composition of lipids, liposomes can be characterized according to size, charge, lamellarity etc. This article provide an overview of liposomes, advantages, disadvantages, mechanism of action, classification, structural composition, preparation as well as evaluation parameters, applications and future aspects.
INTRODUCTION
In the early 1960’s Bangham and colleagues discovered the liposomes which became most widely used drug delivery system. [Vyas SP and Khar RK (2002)]. Liposomes are used as therapeutic tool in tumor targeting, antisense and gene therapy, genetic vaccination, immune modulation, lung therapeutics, fungal infections and skin care and topical cosmetic products. [Vyas SP and Khar RK (2002)]. Liposomes are concentric bilayered vesicles in which an aqueous volume is entirely enclosed by a lipid bilayer membrane made up of natural or synthetic phospholipids. ‘Lipos’ means fat and ‘Soma’ means body. [Patel Chirag et al., (2020)]

Fig.1 : Structure of liposome [Din Fakhar Ud, (2017)]
Advantages of liposomes: [Vyas SP and Khar RK (2002) and Patel Chirag et al., (2020)]
1. Biocompatible , non- toxic , flexiblebiodegradable and non-immunogenic for systemic as well as non-systemic administrations.
2. Selective passive targeting to tumor tissues.
3. Increased therapeutic index and efficiency.
4. Decreased toxicity.
5. Controlled and sustained release.
6. Site avoidance effect.
7. Encapsulation enhances stability.
8. Suitable for hydrophobic, amphiphatic and hydrophilic drugs.
Disadvantages of liposomes: [Patel Chirag et al., (2020) and Maripati S. et al., (2014)]
Following IV administration, liposomes are rapidly removed from the blood by reticulo endothelial system cells and Kupfer cells.
1. Decreased stability and solubility.
2. Shorter biological half life.
3. Increase production cost.
4. Phospholipids may undergo oxidation or hydrolysis.
MECHANISM OF FORMATION
Lipid membrane of liposomes is made up of a bilayer forming amphiphile, cholesterol and a charge generating molecule. [Vyas SP and Khar RK (2002)]
Liposomes are formed upon hydration of the phospholipids (amphiphilic molecules having hydrophilic tail and hydrophobic head). The hydrophobic tail consists of two fatty acid chains with 10-24 carbon atoms and 0-6 double bonds in each chain. The polar end i.e. the hydrophilic head is composed of phosphoric acid bound a to water soluble molecule. [Yadav D. et al., (2017)]
When they are dispersed in aqueous medium they organize themselves to form lamellar sheets where the polar head group faces outwards to the aqueous region and fatty acid groups forms a spherical vesicle like structure facing each other, called as liposomes. The polar region stays in contact with aqueous region and shields the non polar part. [Sharma D. et al., (2018)]
The hydrophilic/hydrophobic interaction between lipid-lipid or lipid-water molecules leads to the formation of bilayer vesicles which arrives at a thermodynamic equilibrium in the aqueous phase. This occurs only when phospholipids are hydrated in water with input of energy like homogenization, shaking, sonication, etc. [Yadav D. et al., (2017) and Sharma D. et al., (2018)]
Important parameters affecting bilayer formation are: [Vyas SP and Khar RK (2002)]
• Hydrophobic interaction along with the amphiphilic nature of the main phospholipid molecules is the reason for bilayer structure of liposomes.
• Due to the high differences in free energy between the hydrophobic and aqueous environment, the bilayer structure is raised to attain the lowest free energy level.
• Super molecular self assemblages can gain maximum stability by forming into vesicles through specific molecular geometry.
CLASSIFICATION
Liposomes are produced by various methods and their nomenclature depends on their method of preparation, special functions or their structural parameters. [Vyas SP and Khar RK (2002)]
TABLE 1 : Classification of liposomes
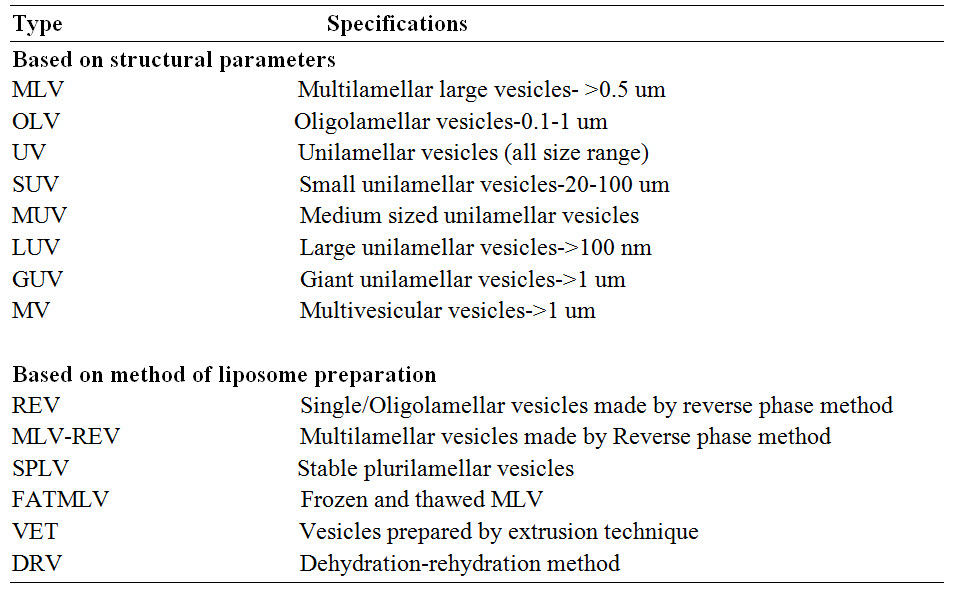

Fig.2: Different types of liposomes
STRUCTURAL COMPOSITION
1) Phospholipids
Phosphatidylcholine (PC) is one of the most commonly used phospholipids in liposome preparation. It can be obtained from both natural and synthetic sources. It consists of a hydrophilic group with a quaternary ammonium moiety choline, which is linked via phosphoric ester to a glycerol. The hydrogen chain of lipid molecules ensures the stability of liposome membrane [Joshi AJ et al., (2014)]. Glycerol containing phospholipids are most commonly used component in liposome formulation [Wu F. et al., (2017)].
Examples of phospholipids are [Deepti SG et al., (2020)]:
• Phosphatidyl choline (Lecithin) – PC
• Phosphatidyl ethanolamine (Cephalin) – PE
• Phosphatidyl serine (PS)
• Phosphatidyl inositol (PI)
• Phosphatidyl glycerol (PG)
2) Sphingolipids
Sphingolipids are a class of lipids that are integral component of both plant and animal cells. [Wu F. et al., (2017)]. Sphingolipids are formed from palmitoyl coA and serine. Cell uses sphingosine to form ceramide. Ceramide are structural units of all Sphingolipids and are formed from long chain of fatty acids and sphingosine. Most commonly used sphingolipids are sphingomyelin, glycosphingolipids. Sphingomyelins are only type of phospholipids which do not have a glycerol backbone [Engelking Larry R et al., (2015)].
3) Sterols
Sterols are cholesterols or derivatives of cholesterols which are used to decrease bilayer fluidity, decrease permeability of water and increase the stability of the bilayer in the biological environment [Rukhsana Yusaf et al., (2014)]. Liposomes without cholesterol rapidly react with plasma protein such as albumin, transferring, and macroglobulin which leads to extract bulk phospholipids from liposomes, thus depleting the outer monolayer of the vesicle causing physical instability [Shashi Kant et al., (2012)]. In the concentration ratio of 1:1 or 1:2, cholesterol is incorporated in phospholipids. Cholesterol enters into the membrane by its hydroxy group facing the aqueous environment and phospholipid bilayer acetyl chain placed parallel to the acyl chain in the middle of the bilayer. By its incorporation lipid bilayer can be changed thus increasing its stability. Both hydrophilic and specific head group interaction ensures high solubility of cholesterol in phospholipids liposomes [Anwekar Himanshu et al., (2011) and Van Hoogevest P. et al., (2011)].
4) Synthetic phospholipids [Shashi Kant et al., (2012) and Gallagher ES et al., (2014)]
Synthetic phospholipids are a type of phospholipids where specific molecular species of polar head groups or fatty acids are introduced by means of chemical synthesis process.
Examples for saturated phospholipids are:
• Dipalmitoyl phosphatidyl choline (DPPC)
• Distearoyl phosphatidylcholine (DSPC)
• Dipalmitoyl phosphatidyl serine (DPPS)
• Dipalmitoyl phosphatidic acid (DPPA)
• Dipalmitoyl phosphatidyl glycerol (DPPG)
Examples for unsaturated phospholipids are:
• Dioleoyl phosphatidyl choline (DOPC)
• Dioleoyl phosphatidyl glycerol (DPOG)
Synthetic phospholipids were basically designed to optimize the drug targeting properties of liposomes.
5) Polymeric materials
In the Hydrocarbon chain, synthetic phospholipid with diactylenic group when exposed to UV leads to formation of polymeric liposomes having higher permeability barrier to encapsulate aqueous drugs. Example: For other polymerizable lipids - lipids containing conjugated diene, methacrylate etc. Also several polymerizable surfactants are also synthesized [Shashi Kant et al., (2012)].
6) Polymeric bearing lipids
Stability of repulsive interaction with macromolecules is governed by repulsive forces. Thus by coating liposome surface with charged polymer the repulsion can be induced. Polymeric bearing lipids are prepared by polymerization of lipid membrane which significantly stabilizes the membrane architecture by directly covalent coupling of adjacent lipid molecule [Shashi Kant et al., (2012)].
Polyethylene oxide polyvinyl alcohol and polyoxazoline are example of non ionic and water compatible polymer having higher solubility during absorption due to hydrophilic segment and hydrophobic part of such copolymer, leads to liposome leakage, so best results can be achieved by covalently attaching polymer to phospholipids. Examples: Diacyle Phosphatidyl Ethnolamine with PEG polymer linked via a carbon at or succinate bond [Gallagher ES et al., (2014)].
7) Cationic lipids
Cationic lipid is a positively charged amphiphile which contains three structural domains: i) a positively hydrophilic head group; ii) a hydrophobic portion composed of a steroid or of alkyl chains; iii) a linker connecting the cationic head group with the hydrophobic anchor [Martin B. et al., (2005)].
Examples: DODAB/C: Dioctadecyl dimethyl ammonium bromide or chloride.
DOTAP: Dioleoyl propyl trimethyl ammonium chloride – an analogue of DOTAP and various others includes various analogues of DOTMA and cationic derivatives of cholesterol [Shashi Kant et al., (2012)].
8) Other substances [Joshi AJ et al., (2014)]
• In case, if the drug is prone to oxidation, various antioxidants are used such as tocopherol, butylated hydroxy toluene.
• Variety of stabilizers are used to form stable liposome
• Preservatives are also used to increase the shelf life of liposomal formulation.
PREPARATION OF LIPOSOMES
Liposomes are mainly prepared two methods via passive loading techniques and by active loading techniques.
Passive loading techniques include three different methods:
I. Mechanical dispersion method
II. Solvent dispersion method
III. Detergent removal method
Passive Loading Techniques
I. Mechanical dispersion method [Vyas SP, Khar RK (2002)]
1. Thin film hydration using hand shaking (MLVs) and non-shaking methods (ULVs)
These methods involves casting of lipids as stacks of films from their organic solution either using flash rotary evaporator under reduced pressure or by hand shaking followed by dispersion of casted films into aqueous solution. Upon hydration the lipids swell and flake off from the round bottom flask’s wall and vesiculate to form MLVs. In hand shaking method, mechanical energy is imparted by manual agitation and in non shaking method energy is imparted by exposing the film to stream of water saturated nitrogen for 15 min. The percentage encapsulation efficiency as high as 30% can be achieved.
2. Micro-fluidization
Micro-fluidization method is also known as micro-emulsification, which is used for large scale production of liposomes [Olgo Poposka et al., (2013)]. It is relatively a new technique which includes the force of the two stream of liposomes suspension colliding with each other under high pressure to reduce the vesicle size. Uniformly hydrated phospholipid suspension (unisized liposomes) is transferred to the reservoir. Through the interaction chamber the liposome suspension is pumped under pressure. Further, to produce smaller and more uniformly sized liposome, the suspension is divided into two streams and then recombined at high velocity in the interaction chamber [Vemuri S. et al., (1990)].
The mean vesicle size of the liposome reduced drastically to 0.1 and 0.2 µm in diameter after three passes through micro fluidizer.

Fig. 3: Liposomes prepared by Micro-fluidization method [Vyas SP and Khar RK (2002)]
3. Sonication
Sonication is the most widely used method for preparation of SUV’s. At higher energy levels, the average size vesicles are further reduced. Here, the MLV’s are exposed to ultrasonic irradiation. [Vyas SP and Khar RK (2002)]
Two types of sonicators are used i.e. a Probe sonicator and a Bath sonicator. Probe sonicator is used for dispersions having energy in small volumes (example- high lipoidal concentration or a viscous aqueous phase). The energy supplied by the probe tip into the lipid dispersion is very high. Bath sonicators are used for large volumes of diluted lipids. [Vyas SP and Khar RK (2002) and Akbarzadeh et al., (2013)]
The prepared SUV’s are purified by ultracentrifugation. Disadvantages of sonication method are: low encapsulation efficiency, metal pollution from probe tip, phospholipid degradation and presence of some MLV’s along with SUV’s. [Maripati S. et al., (2014)]
4. French pressure cell
In this method “uni or oligo lamellar liposomes” of intermediate size of 30-80 nm in diameter are yield depending on the applied pressure. [Vyas SP and Khar RK (2002)]. Under high pressure, passage through a small orifice, dispersion of MLV’S can be converted to SUV’s. In French pressure cell MLV’s dispersion are extruded at about 20,000psi at 45°C. The method is rapid and reproducible. The liposomes formed by this method are larger than sonicated SUV’s. Few drawbacks of this method are high temperature required is difficult to achieve and working volumes are smaller (nearly 50 ml as the max). [V Deepthi et al., (2014) and Argan Nikhil et al., (2012)]

Fig. 4: Liposome prepared by French pressure cell [Vyas SP and Khar RK (2002)]
5. Dried Reconstituted Vesicles (DRV’S)
This method involves freeze drying of a dispersion of an empty SUV followed by its rehydration with aqueous fluid containing the material to be entrapped. [Vyas SP and Khar RK (2002) and Kirby CJ et al., (1980)] Liposomes formed from DRV method are 0.1µm or less in diameter i.e. uni- or oligo-lamellar liposomes. High entrapment of water soluble components and use of mild conditions for preparations and loading of bioactive are the major advantages of this method [Gregoriadis G. et al., (1990)].
6. Freeze Thaw Sonication (FTS) Method [Vyas SP and Khar RK (2002), Maripati S. et al., (2014), Akbarzadeh et al., (2013) Mayer LD et al., (1985) and Ohsawa T. et al., (1985)]
FTS method is an addition to the classical DRV method. SUV’s are rapidly frozen and thawed by sanding at room temperature for 15 min followed by subjection to a sonication for a short duration of time. The unilamellar vesicles are formed due to the fusion of SUV throughout the processes of freezing and thawing. The entrapment efficacy varies from 20% to 30%.

Fig.5 : Liposomes prepared by Dried Reconstituted Vesicle (DRV’s) and Freeze Thaw Sonication (FTS) Method [Vyas SP and Khar RK (2002)]
Membrane extrusion method
Membrane extrusion is a technique in which the liposome suspension is passed through membrane filter of defined pore size in order to reduce the size of liposomes. This technique is used to process LUV’S and MLV’S. The equipment is equipped with a pump that pushes fluid or the preparation through the membrane to accomplish the extrusion process. There are two types of membrane filter for this process, the tortuous path type and nucleation track type. Various types of extruders are Lipex extruder, Extruder®, Avestin liposofastTM 50 etc. Various parameters of extrusion method are applied pressure, number of cycle, pore size influence the mean diameter and size distribution of the prepared liposomes. The process is easy, reproducible inhibit phospholipid degradation which increases the encapsulation efficiency of the liposome preparation. The encapsulated volume is 1-2 liters/mol of lipid. [Vyas SP and Khar RK (2002) and Ong SGM et al., (2016)]
II. Solvent Dispersion Method
1. Ether injection method
A lipid solution is dissolved in ether ethanol mixture or diethyl ether is injected slowly through a narrow needle into an aqueous solution of material to be encapsulated at the temperature of vaporizing the organic solvent or under reduced pressure which subsequently leads to the formation of liposomes. The exposure of the compound to be encapsulated at higher temperature, leads to their degradation which can be prevented by using fluorinated hydrocarbon (Ferons) instead of ether. The efficiency of liposomes formed is relatively low, although the volume encapsulated per mole of lipid remains high 8-17/mol [Akbarzadeh et al., (2013)].
2. Ethanol injection
A rapid injection of an ethanol solution of lipid, through a fine needle into an excess of saline or other aqueous medium. To achieve complete mixing, infusion is done at high rate so that the ethanol is diluted rapidly in water and dispersion of phospholipids molecule occurs throughout the medium. This method yields high proportion of SUV’S (~25), although if the mixture is not throughout enough lipid aggregates and larger vesicles may form [Vyas SP and Khar RK (2002)]. The main advantage of the method is use of non-harmful solvent such as ethanol. The main drawback of the method are heterogeneous population is formed (30-110nm), very dilute liposomes are formed as ethanol forms azeotrope with water thus it is difficult to remove all ethanol and the presence of even low amount of ethanol can lead to inactivation of various biologically active molecule [Dua JS et al., (2012)].

Fig. 6 : Liposomes prepared by Ether and Ethanol Injection Method [Vyas SP and Khar RK (2002)]
3. Reverse phase evaporation method
Similarly to the above injection method, several phospholipids (pure/mixed with cholesterol) can be used in this method [Thakur Varun et al., (2012)]. In a round bottom flask, the lipid mixture is added and by the rotary evaporator the solvent is removed under pressure. The system is purged with nitrogen. The lipid is re-dissolved in the organic phase, in which reversed phase vesicles will be formed [Szoka Francis et al., (1978)]. The two phase system is sonicated until the mixture becomes clear one phase dispersion, also known as inverted miscells. By the help of rotatory evaporator the organic solvent is removed slowly until, inverted miscells are converted into viscous state and gel forms. The gel state collapses at a critical point in this process, and some of the inverted miscells are disturbed. The resulting liposomes are called reverse face evaporation vesicles (REV). High encapsulation rate up to 50% is the main advantage of this method. [Vyas SP and Khar RK (2002) and Sharma D. et al., (2018)].
III. Detergent depletion (removal)
In this, the phospholipids are bought in intimate contact with the aqueous phase via detergents, which associate with phospholipids molecules. The structures formed due this association are called as ‘micelles’. The shape and size of the micelles depends on the chemical nature of the detergent, concentration and other lipids involved. Critical Micelle Concentration (CMC) is the concentration of detergent in water at which micelles begin to form. Below CMC detergent molecules remains in free solution. When concentration of added detergent is increased more amount of detergent is incorporated into the bilayer until the conversion from lamellar to spherical micelles takes place. On further increase in concentration of detergent, the micelles are reduced in size [Vyas SP and Khar RK (2002)].
B. Active (remote) loading
In this method, internalization of preformed liposomes is typically driven by a trans-membrane pH gradient. The pH surrounding the liposome allows some of the drug to remain in unionized form, hence, allowing it to migrate across the bilipid layer. Once, inside the liposomes, the drug becomes ionized and gets entrapped here due to the difference in pH. [Vyas SP and Khar RK (2002)]
Advantages of active loading method over passive encapsulation techniques are-
• A high encapsulation efficiency and capacity.
• A reduced leakage of encapsulated compounds.
• Limited chemical degradation during storage.
• Avoidance of biological active compounds during preparation steps in the dispersion thus reducing safety hazards.
EVALUATIONS
After formulation of liposomes they are evaluated to predict their in vitro and in vivo performances. [Vyas SP and Khar RK (2002), Maripati S. et al., (2014) and Shashi Kant et al., (2012)]
The characterization of liposomes is mainly classified in three categories including physical, chemical and biological parameters. Physical parameters includes size, shape, surface features, lamerallity, phase behavior and drug release profiles. Chemical parameters include studies which prove the purity and potency of different liposomal constituents. Biological parameter helps in establishing the safety and suitability of preparations for therapeutic use.
1) Vesicle shape and lamellarity
Vesicle shape can be determined using various electron microscopic techniques, and can also be used to determine the average particle size. Lamellarity of the vesicle i.e., the number of bilayers present in the liposomes is evaluated using Freeze-fracture electron microscopy and 31P nuclear magnetic resonance analysis.
2) Vesicle size and size distribution
Various techniques are describes in the literature for determination of size and size distribution. These methods include light microscopy, fluorescent microscopy. Electron microscopy, laser Light Scattering, Photon Correlation Spectroscopy, Gel Permeation and Gel Exclusion and Zetasizer. Electron microscopy is the most precise method but hence is very time consuming.
a) Microscopic techniques
i. Optical microscopy: Vesicle size of large vesicles (>1µm) can be determined using Bright field, contrast and fluorescent microscope.
ii. Negative Stain Transmission Electron Microscopy (TEM): The use of negative stain TEM facilitates estimation of the liposome size range at the lower end of the frequency distribution. Negative stains used in TEM analysis are Ammonium Molybdate, Uranyl Acetate and Phosphotungstic acid.
iii. Cryo-Transmission Electron Microscopy Technique (Cryo-TEM): It has been used to evaluate size of the vesicles and surface morphology and also used to characterize liposomal formulations where the drug is loaded by remote loading to ensure their stability. The method involves freeze fracturing of the samples followed by their visualization using TEM.
iv. Freeze Fracture Electron Microscopy: It is mainly used to determine the surface features and lamerallity. It can also be used to calculate true vesicle diameter.
b) Diffraction and Scattering Techniques
i. Laser light scattering: Laser based, quasi-elastic light scattering techniques are useful to analyze the homogenous colloidal particulate populations. This technique is based on the time dependent coherence of light scattered by a vesicle. It can be applied to systems with mean diameter less than 1µm.
c) Hydrodynamic Techniques
These techniques include Gel permeation, Field Flow Fractionation and Ultracentrifuge techniques.
3) Surface charge
Zeta potential and free flow electrophoresis are used to study charge on the vesicle surface. Vesicle surface charge is calculated from the mobility of the liposomal dispersion in a suitable buffer.
4) Encapsulation efficiency
It determines the amount and rate of entrapment of water soluble agents in the aqueous compartment of the liposomes.
APPLICATIONS [Vyas SP and Khar RK (2002), Maripati S. et al., (2014) and Shashi Kant et al., (2012)]
1. Liposomes as protein\drug delivery vehicles:
• Altered bio distribution and pharmacokinetics
• In situ sustained and controlled drug release.
• Enzyme replacement therapy
• Lysosomal storage diseases
• Increased solubilization of drugs
• Altered bio distribution and pharmacokinetics
2. Liposomes in gene diseases:
• Gene and antisense therapy
• DNA vaccination
3. Liposomes as carriers for vaccines.
4. Liposomes as carriers for drugs in oral treatment.
5. Liposomes for topical applications.
6. Liposomes for pulmonary delivery.
7. Liposomes against Leishmaniasis.
8. Liposomes for ophthalmic delivery of drugs.
9. Liposomes in immunology:
• Immune adjuvant
• Immune modulator
10. Liposomes as artificial blood surrogates.
11. Liposomes in bioreactors and enzyme immobilization technology.
12. Liposomes in antifungal, antimicrobial, and antiviral treatments
FUTURE ASPECTS [Rukhsana Yusaf et al., (2014), Gatt S. et al., (1991) and Kuhlencord A. et al., (1992)]
Considering liposomal drug delivery system in the future, novel drugs can be converted into convectional liposomes with enhanced circulation. Delivery of ribozymes and oligonucleotides is also assured in future. Encapsulated allergens and blood based artificial liposomes are the future applicant for development and are used in allergy treatment as desensitizer. Immunotherapy, diagnostic assay and targeted delivery of drugs are three major areas for advancement in future. By using liposomal kit with synthetic composition of lipid bilayer, for the measurement reproducible results can be obtained. Food, cosmetics, nutrition and coating industries etc are different areas of liposomal development in future. Spatial and temporal release of liposome encapsulated drugs at site of action is one of the routes that call for future improvement in treatments.

TABLE 2 : Liposomal product list for commercial use along with their indications [Harshita Gupta et al., (2019)]
CONCLUSION
Liposomes have been identified as tremendously useful carrier systems and tools for targeted drug delivery. Liposomes are achieving clinical recommendations due to their enhanced drug delivery to the diseased locations. Liposomes are of particular interest as intracellular delivery systems for anti sense molecules, ribosome, proteins/peptides, and DNA. The flexible behavior of liposomes and their reduced toxicities are utilized for drug delivery through any route of administration and for any drug or material irrespective of their physiochemical properties. However, based on the pharmaceutical applications and available product, we can say that liposomal drug delivery has a great promise in the future and is sure to undergo further developments.
REFERENCES
1. Akbarzadeh et al. “Nanoscale Research Letters”; 2013, 8:102; Page 4-9 http://Www.Nanoscalereslett.Com/Content/8/1/102.
2. Anwekar Himanshu, Patel Sitasharan, and Singhai A. K (2011); “Liposome- As Drug Carriers”; International Journal of Pharmacy and Life Science; 2(7); 945-951.
3. Argan Nikhil, Harikumar SL, Nirmala (2012); “Topicsl Liposomal Gel: a Novel Drug Delivery System”; IJRPC; 2(2); 383-391.
4. Deepti SG. Available at https://www.pharmatutor.org/articles/liposomes-overview-novel-trend-drug-delivery. Retrieved on March 24, 2020.
5. Din Fakhar Ud (2017); “Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors”; International Journal of Nanomedicine; 12; 7291-7309 Http://Dx.Doi.Org/10.2147/IJN.S146315.
6. Dua JS, Rana AC, Bhandari AK (2012); “Liposome: Method of preparation and applications”; International Journal of Pharmaceutical Studies; 3(2); 14-20.
7. Engelking Larry R (2015); “Textbook of Veterinary Physiological Chemistry”, 3rd Edition. Academic Press, USA; Https://Doi.Org/10.1016/B978-0-12-391909-0.50059-1.
8. Gallagher ES, Mansfield E, Aspinwall CA (2014); “Stabilized Phospholipid Membranes in Chromatography: Toward Membrane Protein-Functionalized Stationary Phases”; Anal Bioanal Chem.; 406(9-10); 2223–2229.
9. Gatt S, Bercovier JH and Barenholz Y. (1991); “Use of liposomes to combat oil spills and their potential application to bioreclamation, in: On Site Bioreclamation, Eds R.E. Hinchee and R.F. Olfenbuttel (Butterworth, Stoneham); 293–312.
10. Gregoriadis G, De Silva H, Florence AT; (1990); International Journal of Pharm.; 65; 235.
11. Harshita Gupta, Harsha Gupta, Suchandra Goswami, Diptendu Goswami(2019) ; "An Updated Review on: Liposomal Drug Delivery System"; in Journal of Advances and Scholarly Researches in Allied Education; 16(6); 702-709. http://ignited.in//J/JASRAE/6/16.
12. Joshi AJ, et al. Liposomes: “Emerging Trends in Novel Drug Delivery with Present and Future Challenges”. IJPBA.2014; 6(2): 3-8.
13. Kirby CJ, Gregoriadis G. (1980); Life Sciences; 27; 2223.
14. Kuhlencord, A, Maniera, T, Eibl, H and Unger, C; “Antimicrob. Agents Chemother.” 36:1992, 1630–1634.
15. Maripati S, Umashankar K, Reddy PJ; “A Review on Liposomes”; International Journal of Research in Pharmaceutical and Nano Sciences;2014; 3(3); 159-169.
16. Martin B, Sainlos M, Aissaoui A, Oudrhiri N, Hauchecorne M, Vigneron JP, Lehn JM, Lehn P (2005); “The Design of Cationic Lipids for Gene Delivery”; Current Pharmaceutical Design; 11(3); 375-394.
17. Mayer LD, Hope MJ, Cullis RP, Janoff AS. (1985); Biochim. Biophys. Acta 817; 193.
18. Ohsawa T, Miura H, Harada K, (1985); Pharm. Bull. 33, 2916.
19. Olgo Poposka, Klopcevska Zoran, Rafajlovaka Vesna (2013); “An Overview: Methods for Preparation and Characterization of Liposomers as Drug Delivery System”; International Journal of Pharm. Phytopharmacological Research; 3(3); 182-189.
20. Ong SGM, Chitneni M, Lee KS, Ming LC, Yuen KH (2016); “Evaluation of Extrusion Technique for Nanosizing Liposomes”; Pharmaceutics; 8(4); 36.
21. Patel Chirag. Available at https://www.pharmatutor.org/articles/liposomes-novel-drug-delivery-carrier. Retrieved on April 27, 2020.
22. Rukhsana Yusaf et al.; “Structural Components of Liposomes and Characterization Tool”; Indo American Journal of Pharma Research.2014:4(08).
23. Sharma D, Ali AAE, Trivedi LR; “An Updated Review On: Liposomes as Drug Delivery System”; Pharmatutor; 2018; 6(2); 50-62.
24. Shashi Kant, Satinder Kumar, Parashar Bharat (2012); “A Complete Review On: Liposomes”; International Research Journal of Pharmacy; 3(7); 10-16.
25. Szoka Francis, Papahadjopoulos D. ¬(1978); “Procedure for Preparation of Liposomes with Large Internal Aqueous Space and High Capture by Reverse Phase Evaporation”; Processings of the National Academy of Sciences of America; 75(9); 4194-4198
26. Thakur Varun, Arora Sonia, Prashar Bharat, Patil Vishal (2012); “Niosomes and Liposomes- Vesicular Approach towards Transdermal Drug Delivery”; International Journal of Pharmaceutical and Chemical Sciences; 1(3); 981-993.
27. V Deepthi and AN Kavithr (2014); “Liposomal Drug Delivery System-a Review”; RGUHS J Pharma Sci.; 4(2); 47-56.
28. Van Hoogevest P and Wendel A (2011); “The Use of Natural and Synthetic Phospholipids as Pharmaceutical Excipients”; European Journal of Lipid Science and Technology; 116(9); 1088-1107.
29. Vemuri S, Yu Ching D, Wangsatorntanakun V, Roosdorp N (1990); “ Large-Scale Production of Liposomes by a Microfluidizer”; Drug Dev Ind Pharm; 16(15); 2243–2256.
30. Vyas SP, Khar RK (2002), “Targeted and Controlled Drug Delivery”, 1st Edition. CBS Publishers; New Delhi.
31. Wu F, Bhansali SG, Tamhane M, Kumar R., Vathy LA, Ding H, Yong K, Bergey EJ, Prasad PN (2012); “Noninvasive Real-Time Fluorescence Imaging of Lymphatic Uptake of BSA-IR Dye 680 Conjugate Administered Subcutaneously in Mice”; Morris MEJ Pharm Sci.; 101(5); 1744-54.
32. Yadav D, Sandeep K, Pandey D, Dutta RK (2017); “Liposomes for Drug
Delivery”; J Biotechnol Biomater 7: 276.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









