About Authors: Shoeib Afroz Mohammad,
M.Pharm (Pharmacology),
Vaagdevi College of Pharmacy, Kakatiya University
Reference ID: PHARMATUTOR-ART-1087
BACKGROUND:
By the 1930s, scientists had isolated and determined the structure of the steroid hormones and found that high doses of androgens, estrogens or progesterone inhibited ovulation. The first oral-contraceptive formulations marketed in the United States, in 1960 and1961, contained 2 to 5 times as much estrogen and 5 to 10 times as much progestin as the oral contraceptives now in use. Since introduced in May of 1960, these pills have provided reliable contracep¬tion for millions of woman throughout the world.They were given in a regimen consisting of 21 active tablets containing estrogen and progestin followed by 7 days of placebo tablets (21/7 regimen). The 7 days of placebo was designed for menses to occur during that time. The use of these high-dose formulations was linked to increased risks of ischemic stroke, myocardial infarction, and pulmonary embolism in healthy young women. The estrogen and progestin were reduced rapidly during the 1960s and 1970s because of concern about safety and because the reduction of the doses did not reduce the contraceptive effectiveness.The reductions in the dose of estrogen are believed to have decreased the risk of venous thrombosis. The combination estrogen–progestin oral contraceptives that are now on the market contain estrogen at doses ranging from 20 to 50 μg of ethinyl estradiol or, uncommonly, mestranol. Currently there are over 70 different brands on the market. 1
[adsense:336x280:8701650588]
These estrogens are combined with any of several different progestins, which may be given at the same dose every day (“monophasic”) or at varying doses according to the phase of the cycle (“biphasic” or “triphasic”) to mimic more closely the production of progesterone during the normal menstrual cycle.
DEFINATIONS:
Oral contraceptives are medications taken by mouth for the purpose of birth control. “Active pills” refer to the pills in the package that contain hormones. “Inactive” or “placebo” pills are members of the pill package that do not contain any hormone. Their presence in the pack is to help a women stay on schedule taking her pills. Inactive pills always have a different color from the active pills. During the days that the inactive pills are taken, a women will likely have a period. For years, all pill packs had 21 days of active pills and 7 days of inactive pills = 28 pills creating a monthly menstrual cycle.
Biphasic pills – 0.035mg EE + low dose P – 10 days 0.035mg EE + high dose P - 11 days Triphasic pills – Triquilar: l-norgestrel 0.05mg (6days), 0.075mg (5days), 0.125mg (10days) + constant 0.03mg EE 2
CLASSIFICATION:
Recently, oral contraceptives have been classified by some according to “generation” (first, second, third, and most recently, fourth generation). These terms sometimes refer to the timing of the introduction of a product (given both the dose of estrogen and the type of progestin), sometimes refer to the timing of the market introduction of the progestin, sometimes refer to the structure of the carbon ring from which the progestinis derived (estrane or gonane).The various types of oral contraceptives available are:
1) Female: Two types of female oral contraceptive pill are widely available:
• The combined oral contraceptive pill contains oestrogen and a progestogen, and is taken once per day.
• The progestogen only pill contains only progestogen, and is also taken once per day.
Other types of female oral contraceptive are experimental or only available in limited areas:
• Mifepristone is an antiprogestogen which has been used as a daily oral contraceptive in investigational clinical trials. 3
• Ormeloxifene (also known as Centchroman) is a selective oestrogen receptor modulator which is taken one to two times per week. Ormeloxifene is approved as an oral contraceptive only in India.
2) Male: Male oral contraceptives are currently not available commercially, although several: possibilities are in various stages of research and development
Chemical Structure of Oral Contraceptives
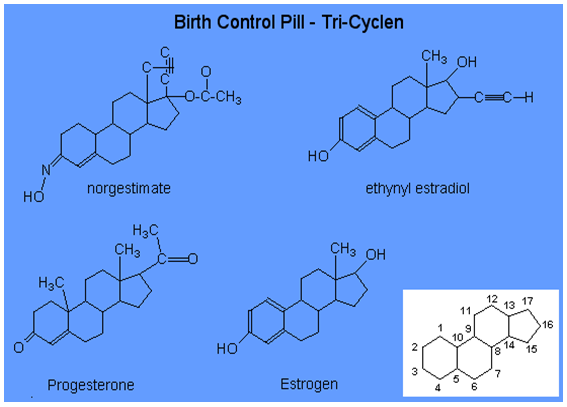
Benefits of oral-contraceptive use:
1) In Ovarian Cancer
The risk of ovarian cancer is reduced by atleast half among women who use oral contraceptives, including those who use low-estrogen formulations. The reduction in risk occurs after relatively short term use (5 years) and persists for 10 to 20 years after use has been discontinued. This benefit extends to women with a family history of ovarian cancer and women with a mutation in the BRCA1 or BRCA2 gene. The suggested mechanism for this effect is the suppression of ovulation.
2) In Endometrial Cancer
Older formulations of oral contraceptives, which contained higher doses of estrogen, reduce the risk of endometrial cancer, presumably by progestin mediated suppression of estrogen-induced proliferation of endometrial cells.
[adsense:468x15:2204050025]
3) In Acne:
Randomized, double-blind, placebo-controlled trials show substantial reductions in the severity of acne among both patients given oral contraceptives and patients given placebo, but the patients who received oral contraceptives had greater improvement.
4) In Menstrual Disorders, Loss of Blood, and Anemia:
One randomized, double-blind, placebo-controlled trial of low-dose oral contraceptives showed that their use reduces the severity of dysfunctional uterine bleeding. Oral-contraceptive use decreases menstrual blood flow and is associated with a reduced prevalence of anemia and increased hemoglobin concentrations in anemic women.
COMBINED ORAL CONTRACEPTIVES(COCP):
The combined oral contraceptive pill (COCP), often referred to as the birth-control pill or simply "the Pill", is a birth control method that includes a combination of an estrogen (oestrogen) and a progestin (progestogen). When taken by mouth every day, these pills inhibit female fertility.
Formulations:
Oral contraceptives come in a variety of formulations. The main division is between combined oral contraceptive pills, containing both estrogen and progestins and progestin only pills. Combined oral contraceptive pills also come in varying types, including varying doses of estrogen, and whether the dose of estrogen or progestin changes from 1 week to the next. 4
• Extended-Cycle Oral Contraceptives: Two types of extended-cycle oral contraceptives are available in the United States. One is designed to deliver 24 days of estrogen and progestin followed by 4 days of placebo (24/4 regimen). Currently, two products are FDA-approved for a 24/4 regimen: ethinyl estradiol 20 µg-drospirenone 3 mg and ethinyl estradiol 20 µg-norethindrone 1 mg. The other type of extended-cycle oral contraceptive (84/7) contains 84 days of estrogen and progestin followed by 7 days of either placebo or very low-dose estrogen (10 µg/day).
• Continuous-Cycle Oral Contraceptives: the continuous-cycle pill containing ethinyl estradiol 20 µg-levonorgestrel 90 µg.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Newer Contraceptives Regimen:
These new oral contraceptives are given in the following regimens:
24 days followed by placebo for 4 days (24/4),
84 days followed by placebo for 7 days (84/7), or continuously (without placebo).
These agents contain
• ethinyl estradiol 20 µg-drospirenone 3 mg (24/4);
• ethinyl estradiol 20 µg-norethindrone 1 mg (24/4);
• ethinyl estradiol 30 µg-levonorgestrel 150 µg (84/7);
• ethinyl estradiol 30 µg-levonorgestrel 150 µg (84/7) with very low-dose ethinyl estradiol (10 µg/day) for 7 days; and
• ethinyl estradiol 20 µg-levonorgestrel 90 µg continuously 5
Mechanism of action:
Combined oral contraceptive pills were developed to prevent ovulation by suppressing the release of gonadotropins. Combined hormonal contraceptives, including COCPs, inhibit follicular development and prevent ovulation as their primary mechanism of action. Progestagen negative feedback decreases the pulse frequency of gonadotropin-releasing hormone (GnRH) release by the hypothalamus, which decreases the release of follicle-stimulating hormone (FSH) and greatly decreases the release of luteinizing hormone (LH) by the anterior pituitary. Decreased levels of FSH inhibit follicular development, preventing an increase in estradiol levels.
Progestogen negative feedback and the lack of estrogen positive feedback on LH release prevent a mid-cycle LH surge. Inhibition of follicular development and the absence of a LH surge prevent ovulation. Estrogen negative feedback on the anterior pituitary greatly decreases the release of FSH, which inhibits follicular development and helps prevent ovulation. 6
A secondary mechanism of action of all progestagen-containing contraceptives is inhibition of sperm penetration through the cervix into the upper genital tract (uterus and fallopian tubes) by decreasing the amount of and increasing the viscosity of the cervical mucus.
Other possible secondary mechanisms have been hypothesized. One example is endometrial effects that prevent implantation of an embryo in the uterus. Some pro-life groups consider such a mechanism to be abortifacient.
Effectiveness:
The effectiveness of COCPs, as of most forms of contraception, can be assessed two ways. Perfect use or method effectiveness rates only include people who take the pills consistently and correctly. Actual use, or typical use effectiveness rates are of all COCP users, including those who take the pills incorrectly, inconsistently, or both. Rates are generally presented for the first year of use. 7 Most commonly the Pearl Index is used to calculate effectiveness rates, but some studies use decrement tables. COC’s are about 98-99% effective if taken every day as directed.The typical use pregnancy rate among COCP users varies depending on the population being studied, ranging from 2-8% per year. The perfect use pregnancy rate of COCPs is 0.3% per year.
COCPs provide effective contraception from the very first pill if started within five days of the beginning of the menstrual cycle (within five days of the first day of menstruation). If started at any other time in the menstrual cycle, COCPs provide effective contraception only after 7 consecutive days use of active pills, so a backup method of contraception must be used until active pills have been taken for 7 consecutive days. COCPs should be taken at approximately the same time every day.
Contraceptive efficacy may be impaired by:
1) missing more than one active pill in a packet, 2) delay in starting the next packet of active pills (i.e., extending the pill-free, inactive or placebo pill period beyond 7 days), 3) intestinal malabsorption of active pills due to vomiting or diarrhea, 4) drug interactions with active pills that decrease contraceptive estrogen or progestogen levels.
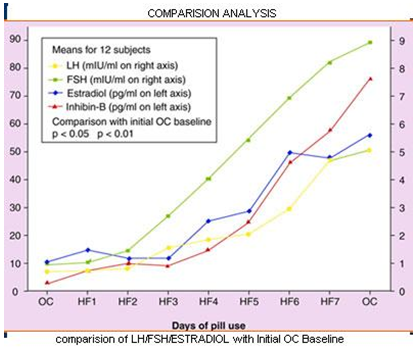
Comparision Of LH/FSH/ESTRADIOL with Initial OC baseline:
Choice of pill formulation:
For younger women without any special health or skin problems it probably makes very little difference which low dose oral contraceptive is prescribed. The presence of conditions such as epilepsy, acne or hirsutism, and also cost, influence the choice of pill. For the majority of women triphasic formulations offer no benefit and may increase the likelihood of premenstrual symptoms, breast tenderness, or in a minority, dysmenorrhoea. For women who develop breakthrough bleeding on low dose monophasic pills a triphasic pill may improve cycle control. 8
Choice of an oral contraceptive in special patient groups:
1) History or strong family history of venous thromboembolism:
Women with no risk factors for VTE may be prescribed any combined COC containing 35 μg or less of ethinyloestradiol. Women with a history of VTE, thrombophilias or multiple risk factors for VTE can use progestogen only methods or alternative methods of contraception. In nonsmoking women over the age of 40 years who carry an increased risk of VTE on account of age, and especially if they are obese, it is wise to prescribe a 20 μg ethinyloestradiol/100 μg levonorgestrel combination preparation initially. If the woman develops side effects on this preparation then changing to a higher levonogestrel combination or using the third generation progestogens is warranted.
2) Adolescents:
Once an adolescent has commenced menstruating COCs can be prescribed. Adolescents shouldbe prescribed a monophasic formulation as they aresimpler to use. For adolescents it is particularly important to prescribe a pill which provides reasonably effective cycle control. Adolescents seem to be less tolerant of breakthrough bleeding than older women and tend to cease taking the pill if this occurs. Clinically, cycle control appears to be better with norethistrone formulations which also have a beneficial effect on acne but may increase the incidence of absence of withdrawal bleeding.
3) Women with epilepsy:
With the exceptions of valproate sodium and clonazepam all the older anticonvulsant drugs induce enzymes in the liver that increase the rate of metabolism of ethinyloestradiol. Women who are using enzyme inducing drugs should use 50 μg
oestrogen pills. If breakthrough bleeding occurs in the second cycle, a double dose of a 35 μg combined pill or a combination of a 30 μg and 50 μg COC can be used. 9
4) Women with acne:
Many women find acne improves when any combined pill is used. However, a number of women find acne worsens when using a levonorgestrel formulation. For these women using a desogestrel, gestodene or cyproterone acetate formulation usually improves the condition. For women who cannot afford the newer pills a formulation containing 500 μg norethisterone, which is less potent and less androgenic than levonorgestrel, usually results in improvement of acne. The low dose tetracyclines used in the management of acne do not reduce the efficacy of the pill. For women with hirsutism the cyproterone acetate formulation is most suitable as this progestogen binds to androgen receptors blocking the action of testosterone, the cause of hirsutism. 10
5) Women over the age of 35 years:
women over the age of 35 years who are nonsmokers can use a low dose COC until the menopause. Continuing oral contraceptive use until the menopause may have considerable benefit, as women in their 40s often develop bleeding problems either due to fibroids, adenomyosis or dysfunctional uterine bleeding. The COCs offer an effective method for controlling such bleeding problems until the menopause 11 and COCs are also the best way for controlling hot flushes and other symptoms of the perimenopause, since it is often difficult to tailor hormone replacement therapy (HRT) to a woman’s naturally occurring cycle.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Best time to start COCs:
Recommendations: COCs may be started any time you can be reasonably sure that the woman is not pregnant, for example, during the 7 days which begin with the onset of
menses (days 1 through 7 of the menstrual cycle).
Rationales: Starting within the first 7 days lowers the possibility of beginning the pill while she is already pregnant (although there is the possibility that the client is pregnant and implantation bleeding has been mistaken for menses).
Benefits:
• Relief of menstrual cycle symptoms including cramps
• Improved acne
• Protection against ovarian and uterine cancer
• Decreased anemia
• Decrease in Cyclic migraines
• Decrease or no PMS, anxiety, and depression
• No periods and lighter every 4 months
• Decrease of occurrence ovarian cysts
• Decrease Fibrocystic Breast Disease
• Less likely to develop Pelvic Inflammatory Disease (PID)
• Reduction in menstrual volume
• Reduction in menstrual exacerbation of seizure disorders, asthma, porphyria. 12
Common side effects of COC’s:
1) Nausea: Some women may experience mild nausea when starting COC’s. Taking the pill at night or with food may help.
2) Breast tenderness or enlargement: This may occur in about 30% of women.Generally this improves as your body adjusts over the first few months.
3) Headaches: This is the most common seen side effect.
4) Unscheduled spotting or breakthrough bleed¬ing: Common the first 3 months of starting COC’s, up to 50%of users may experience this. By the 3rd pack of pills, 90% of women are no longer experiencing spotting. Some women may notice some mild menstrual cramps with the spotting. 13
5) Missed periods or amenorrhea: Sometimes a woman who has taken all her pills correctly will not get her period. This can happen for a variety of reasons ( stress, illness, travel, rarely thyroid or other hormonal issues). A urine pregnancy test is advised before starting a new pack of pills.
6) Weight gain: 3 placebo-controlled randomized clinical studies have demonstrated COC’s do not cause weight changes. However, some women may react to the hormones with more mild fluid retention of some tissue around breasts and hips.
7) Mood changes: depressive feel¬ings or irritability.
8) Decrease in sex drive: The hor¬mones in COC’s may cause a decrease in sex drive in some women.
9) Changes in vaginal discharge: A slight increase in the amount of discharge may occur in some women.. Others may notice less lubrication with intercourse.
10) Contact lens wearer: Rarely, women who wear contacts may notice some visual changes or change in lens tolerance.
Other side effects:
1) Pregnancy: COC’s do not affect future fertility, risk of miscarriage, or birth defects.
2) High Blood Pressure: While most studies show that today’s COC’s have little impact on blood pres¬sure, one study suggested blood pressure could rise. We like to screen our patients when starting COC’s, a few months after starting, and then at their an¬nual visits.
3) Liver tumors: COC’s have been associated with an increased risk of forming benign liver tumors. This is a very rare occurrence, but you should contact your provider if you develop upper abdominal pain. Additionally, gallstones, which can form in the gall-bladder have a slightly increased risk of developing in women taking COC’s, especially with in women with a family history prone to gallstones. 14
4) Breast Cancer Risk:
A well -studied question, the literature suggests use of COC’s has little if any effect on the risk of breast cancer.
5) Cervical Cancer Risk:
The risk of developing this type of cancer is slightly increased in COC’s users. Fortunately, routine Pap smears are an excellent cancer screening tool.
6) Yasmin, Yaz, Ocella users
This group of pills can cause an elevated potassium in women who take certain blood pressure medica¬tions or medications like: Advil, Motrin, ibuprofen, Aleve, Naproxyn or other prescription nonsteroidal anti-inflammatory medications ( not Tylenol) on a continuous daily basis.
7) Oral contraceptives may influence coagulation, increasing the risk of deep venous thrombosis (DVT) and pulmonary embolism (PE), stroke and heart attack.
Oral Contraceptives and Venous Thromboembolism:
Women who use combined oral contraceptives containing estrogen and progestogen have an associated two- to eight-fold increased risk of venous thromboembolism. Therefore, the associated risk of venous thromboembolism should be taken into account when selecting an oral contraceptive preparation to be used.
• Oral contraceptives containing levonorgestrel is associated with the lowest risk of venous thromboembolism compared with oral contraceptives containing other progestrogens, including preparations containing cyproterone acetate and drospirenone.
• The risk of venous thromboembolism in women taking oral contraceptives could be reduced further by lowering the dose of estrogen from 30 to 20 µg.
• The interaction between individual types of oral contraceptive preparations and other risk factors need to be evaluated. 15
The warning signs of a blood clot spell out the acronym ACHES:
A Abdominal pain
C Chest pain ( also shortness of breath)
H Headaches (especially those that are new, associated with dizziness, fainting, numbness or weakness in extremities)
E Eye problems( blurred vision or loss of vision)
S Severe leg pain (or redness/swelling in calves or thigh).
SIDE EFFECTS (Should decrease or resolve after 3 packages):
The following side effects that should resolve after 3 packages of intake are:Nausea – take with food or take your pill at bedtime
• Light vaginal bleeding between periods–take your pill at the same time everyday and take only 3 inactive pills every 4 months to avoid spotting.
• Tender/enlarged breasts
• Weight gain
• Missed period
• Headache
• Depression
• Bloating
• Mood Changes
• Fatigue
Serious side effects:
The following danger signals should be watched carefully.
• Sharp or rushing chest pain or coughing blood;
• Shortness of breath;
• Unusual swelling or pain in the legs or arms;
• Sudden severe headaches;
• Eye problems such as blurred vision or double vision or loss of vision;
• Severe pain in the stomach or abdomen;
• Yellowing of the skin or eyes;
• Severe depression;
• Unusually heavy bleeding from the vagina;
• New lump in your breast.
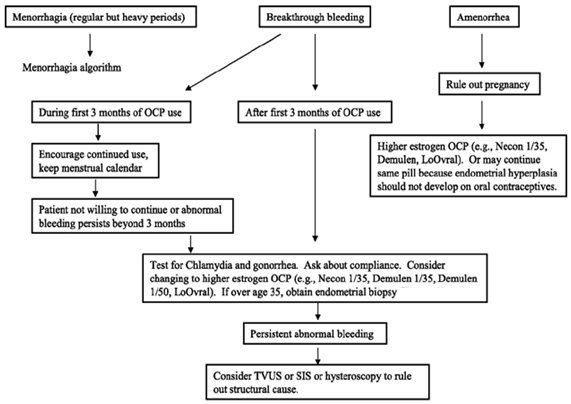
Care to be taken in Amenorrhea and Breakthriough Bleeding:
How To Take:
• If you have chosen to have a monthly withdrawal bleed, take one “active” pill for 3 weeks (21 days), then one week of “inactive, placebo, or sugar” pills. Your withdraw bleed should occur during this time.
• If you have chosen to cycle with a withdrawal bleed 3 times per year, take three weeks or 21 days (“active pills”) then directly start a new package. Repeat this until the fourth package. At that time you need to take three days of inactive (“sugar” or “placebo”) pills to have a withdrawal bleed. Expect a very light bleed. After your 3-day bleed start a new package of pills. Repeat this process. 16
• If you have chosen to have 1 withdrawal bleed a year, then take active pills only until the end of the year (for example, just before your annual exam). At that time take 3 days of inactive pills. You may or may not have bleeding at this time. On the 4th day, start a new pack of active pills.
• If you’ve chosen to have NO withdrawal bleed, do not take the inactive pills ever.
Medicines That Effect My COC’s(Drug Interactions):
The following medicines DECREASE the effective¬ness of COC’s: Topamax, Lamictal,Tegretol, Nevi¬rapine, Trileptal, Phenobarbital, Dilantin, Mysoline, Rifampin, St. John’s Wort, Provigil, Ethosuximine, Griseofulvin, Troglitazone, Vigabatrin .
Some drugs like rifampicin,barbiturates, phenytoin and carbamazepine reduce the effect of the Pill and can cause breakthrough bleeding, or increased chance of pregnancy.In addition cautions are given about broad spectrum antibiotics, such as ampicillin and doxycycline, which may cause problems "by impairing the bacterial flora responsible for recycling ethinylestradiol from the large bowel".The traditional medicinal herb St John's Wort has also been implicated due to its upregulation of the P450 system in the liver. 17
Drugs whose effects may be changed by taking COC’s :
If you are taking any medications for these medical problems and want to begin taking OCPs, you should talk to your health care provider to decide whether any changes need to be made. The drugs include:
• Drugs for seizures or bipolar disorders (e.g. lamotrigine-Lamictal),
• Drugs for blood clotting disorders,
• Antidiabetic drugs,
• Drugs for high blood pressure,
• Anxiolytic drugs,
• Anti depressants,
• Drugs for immune suppression,
• The effects of alcohol and caffeine may be enhanced in women taking OCPs.
Other contraindications to COC use:
• Existing or history of cardiovascular or cerebrovascular disease
• Diabetes with circulatory problems
• Complicated valvular heart disease
• History of thromboembolism
• Severe liver disease
• Breast cancer
• Uncontrolled hypertension
• Focal migraine
• Women >35 years smoking >15 cigarettes per day
• Prolonged immobilisation
• Malabsorption syndrome.
Who Should Not Take the Pill?
Some women should not take the pill. If you have any of the following conditions, the pill might not be right for you:
• History of heart attack or stroke
• Blood clots in the legs, lungs, or eyes
• Chest pain
• Cancer of the breast, uterus, cervix, or vagina
• Vaginal bleeding with no cause
• Liver problems (jaundice, tumors) during pregnancy or during prior use of the pill
• Suspected or known pregnancy
Also, women who take the pill and smoke and those over age 35 have an increased risk of developing blood clots and blockage of blood vessels. These conditions can lead to a heart attack or stroke, with serious injury or death. 18
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
PROGESTOGEN ONLY PILL (MINI PILL):
For women in whom oestrogens are contraindicated, not tolerated or do not want to take a COC the progestogen only pill (minipill) is an often overlooked alternative. There are relatively few contradictions to the progestogen only pill and these include malabsorption syndromes, undiagnosed vaginal bleeding, previous ectopic pregnancy (because if pregnancy does occur with a progestogen only pill there may be a greater incidence of tubal pregnancy) and severe liver disease.19
Family Tree of Contraceptive Progestins
* Synthetic progestins traditionally used in oral contraceptives can be classified as those that are structurally related to progesterone or testosterone.
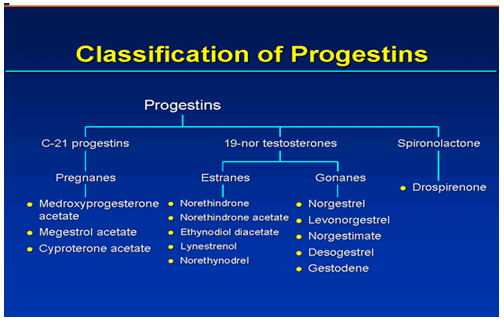
* Progestins that are structurally related to progesterone include progesterone itself and medroxyprogesterone acetate compounds that have 21 carbons.
* Progestins structurally related to testosterone are structural derivatives of testosterone and are not synthesized from the hormone. Removal of the methyl group from the testosterone molecule produces norethindrone, a compound with high progestational activity, high oral activity, and almost no androgenicity.
* Adding another methyl group forms an ethyl group and produces the compound norgestrel, which has even greater progestational activity than norethindrone.
* Another classification of progestins uses the terms gonane or estrane and is based on the number of carbons; gonanes have 17 and estranes have 18.
* Chemical derivatives of levonorgestrel (gonanes) and norethindrone (estranes) are the progestin components of oral contraceptives. Levonorgestrel derivatives include desogestrel, norgestimate, and gestodene .Norethindrone derivatives include norethindrone acetate, ethynodiol diacetate, and lynestrenol .
* Drospirenone is a “new” progestin derived from spironolactone. It has biochemical and pharmacologic profiles similar to endogenous progesterone, including antimineralocorticoid and antiandrogenic properties.
Pharmacologic Effects of Progestins as Contraceptives
These include inhibiting ovulation by suppressing the function of the hypothalamic-pituitary-ovarian axis; modifying the subsequent pituitary surge of luteinizing hormone and follicle-stimulating hormone; slowing transport of the ovum through the fallopian tubes, which limits the time available for fertilization; thickening cervical mucus, which impedes sperm transit; and inhibiting the activation of spermatic enzymes required for ovum penetration (capacitation). 20
Suppression of ovulation is not the main mechanism of action of progestin when used as an oral contraceptive because some women continue to ovulate while using progestogen-only pills. When used as an emergency contraception regimen, the main mechanism of action is the inhibition of ovulation.
Candidates for Progestin-Only Oral Contraceptives:
Candidates for progestin-only pills include women with contraindications for the estrogen component of combination oral contraceptives. The low doses of progestins used in progestin-only pills are not associated with an increased risk of venous thrombosis or cardiovascular disease. As such, these agents are considered to be safe in women with a history of thrombotic disease or other cardiovascular risk factors.
In fact, the World Health Organization and other professional medical organizations endorse progestin-only oral contraceptives for lactating women over combination oral contraceptives. Although combination oral contraceptives may have a negative impact on the quantity and quality of breast milk, progestin-only oral contraceptives have minimal effects on milk production and may even increase production by increasing the release of prolactin. Although progestins can be detected in breast milk, the levels do not affect an infant’s growth or development.
Drospirenone-containing Oral Contraceptives:
COCs that contain the antimineralocorticoid drospirenone (Yasmin, Yaz; 3 mg drospirenone) as the progestin component may decrease the bloating and water retention that commonly occur with COC use. Drospirenone may cause potassium retention, however, leading to hyperkalemia. 21
Norethindrone: Plasma Levels After a Single Oral Dose:
Measurement of norethindrone (1000 μg) plasma levels after a single oral dose indicate that high levels of norethindrone (~14 ng/mL) occur within the first hour. However, norethindrone levels fall precipitously to undetectable levels—below 1 ng/mL at 24 hours—due to rapid distribution and elimination. This finding underscores the importance of consistency when using progestin-only pills; women who do not ingest their next dose at approximately the same time every day increase their risk of an unintended pregnancy due to inadequate levels of the hormone. Serum progestin may also be affected by individual absorption/metabolism and concomitant medications (e.g., griseofulvin and rifampin).
Importance of Correct and Consistent Use of Progestin-Only Pills
With correct use, 5% of women using a progestin-only oral contraceptive will experience an unintended pregnancy during the first year of use. This efficacy rate is comparable to combination oral contraceptives. Correct and consistently timed use by the patient are paramount to the efficacy of progestin-only pills, because plasma levels fall to baseline levels 24 hours after ingestion.If a dose is more than 3 hours late, back-up contraception should be used for 48 hours in order to avoid an unintended pregnancy. Efficacy may be improved by scheduling pill ingestion close to the expected time of intercourse. For instance, if bedtime is the likely time of intercourse, the optimal time of ingestion may be late afternoon in order to ensure the benefits of progestin-related effects on cervical mucus which peak at 4 hours after ingestion.22
Menstrual Irregularities with Use of Progestin-Only Pills:
A 1982 randomized, double-blind trial conducted by the World Health Organization evaluated menstrual effects in 296 users of progestin-only pills. During the first 3 months of use, an average of 53% of users reported frequent bleeding, 23% reported prolonged bleeding, 14% reported irregular bleeding, and 6% reported amenorrhea. During cycles 10, 11, and 12, 74% of users reported frequent bleeding, 26% reported prolonged bleeding, 11% reported irregular bleeding, and 2% reported amenorrhea. Approximately 25% of pill users withdrew from the study because of bleeding disturbances.
A comprehensive review of studies on users of progestin-only pills found that bleeding disturbances are common and are the most frequent reason for discontinuation. Bleeding disturbances are reported less frequently by breastfeeding women who use progestin-only pills, most likely because of lactational amenorrhea.
Emergency Contraception:
Emergency contraception (also called “the morning-after pill”) is higher doses of certain OCP hormones, progestins (Plan B). It is given as soon as possible within 120 hours of intercourse without effective contraception. Emergency contraception is meant for emergency use only and is not an effective method of birth control for routine use. If you are taking OCPs and have intercourse without using a backup method or your backup method fails during a time when you should be using a backup method, you should consider emergency contraception.
Three different emergency contraceptive methods are safe, simple, and widely available in the United States. These are:
(1) ordinary combined oral contraceptives containing ethinyl estradiol and levonorgestrel taken in a higher dose for a short period of time and started within a few days after unprotected intercourse;
(2) levonorgestrel-only tablets used similarly; and
(3) copper-bearing intrauterine devices inserted within approximately 1 week after unprotected intercourse
Plan B™ : Progestin-Only Emergency Contraception:
Plan B™, a prepackaged progestin-only contraceptive for emergency contraception, was approved by the United States Food and Drug Administration (FDA) in 1999.
Plan B™ emergency contraception consists of 2 tablets of levonorgestrel 0.75 mg. One tablet is taken within 72 hours of unprotected intercourse, the other 12 hours after the first dose. Alternatively, the 2 tablets can be ingested at the same time. There is evidence to indicate that emergency contraception can be used up to 120 hours after unprotected intercourse, but the efficacy decreases. 23
Return of Fertility:
The long-term effect on future fertility of women taking extended- or continuous-cycle oral contraceptives is unknown and warrants further investigation. Most experts do not anticipate a difference in fertility rates, especially with the extended-cycle regimens, because withdrawal bleeding occurs with these. Less is known about the effects on fertility with continuous-cycle regimens. However, a recent abstract reported that most (99%) women who took ethinyl estradiol 20 µg-levonorgestrel 90 µg continuously for 1 year and experienced amenorrhea had a spontaneous return of menses within 90 days after discontinuation of use.
New contraceptive pills:
1) Dienogest is another new progestogen which
binds almost exclusively to the progesterone receptor, has anti-androgenic activity and does not bind to sex hormone binding globulin
2) Drospirenone, an analogue of spironalactone, more closely resembles natural progesterone than the nortestosterone derivatives
3) A Progestogen only pill containing 65 μg gestodene, a dose which suppresses ovulation
4) Ethinyloestradiol 15 μg/gestodene 65 μg in 24 active pills with only four pill free days.
Chewable Oral Contraceptive:
Femcon Fe contains 35 mcg ethinyl estradiol and 0.4 mg norethindrone in a spearmint-flavored pill that can be swallowed or chewed. The tablet should be followed immediately by a full 8 oz. of liquid. The proposed advantage of this product is ease of administration. The seven brown tablets in the HFI contain 75 mg ferrous fumarate.
New and Emerging Contraceptive Options:
Advances in contraceptive technology are providing women with an expanding array of contraceptive options, offering the promise of further improvements in acceptability, efficacy, and safety. In addition to new oral contraceptives, such as Yasmin, alternative delivery routes for hormonal contraception are being actively investigated. Long-acting delivery systems, including implants, injectables, patches, vaginal rings, and intrauterine devices, are available or being developed. The goal of these long-acting systems is to deliver low doses of contraceptive hormones continuously to reduce adverse effects and improve adherence while maintaining efficacy. Other formulations under development may further improve patient acceptance and adherence, adverse effect profile, and affordability
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Counseling on Proper Use:
When counseling on COC use, it is important to review the purpose of therapy (i.e., contraception, menstrual cycle control, noncontraceptive benefits) as well as the various benefits and risks of COCs. Backup methods (e.g., condoms, foam) should be available at all times in case the patient runs out of pills, misses doses, experiences a serious adverse effect, or needs protection from STDs. Women may start taking pills on one of the two following schedules.
• Start on the first day of menstrual bleeding. (The "day-one starter" regimen prevents ovulation in the first cycle, eliminating the need to use an alternative backup method for contraception during the first cycle.)
• Start on the first Sunday after menstrual bleeding begins. (This "Sunday starter" regimen prevents periods on weekends, but an alternative backup method is necessary for the first 7 days of pill use).
Missed Pill Recommendations:
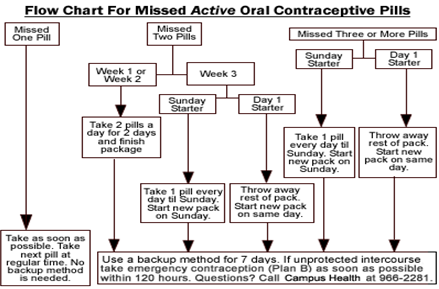
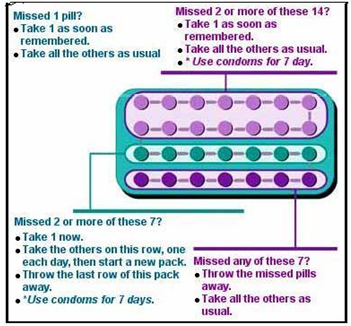
Place in Therapy:
Extended- and continuous-cycle oral contraceptives may be useful for women who desire less frequent scheduled withdrawal bleeding. However, these women should be counseled about the increased frequency of breakthrough bleeding and spotting with these new regimens compared with traditional 21/7 regimens. Some studies have shown that extended- or continuous-cycle oral contraceptives may be useful for women during perimenopause and those with endometriosis, menorrhagia, hormone withdrawal symptoms (e.g., menstrual migraine), or PMDD.
Use and packaging:
Half-used blister pack of LevlenED Combined oral contraceptive pills should be taken at the same time each day. If one or more tablets are forgotten for more than 12 hours, contraceptive protection will be reduced. Most brands of combined pills are packaged in one of two different packet sizes, with days marked off for a 28 day cycle. For the 21-pill packet, a pill is consumed daily for three weeks, followed by a week of no pills. For the 28-pill packet, 21 pills are taken, followed by week of placebo or sugar pills.. There are also two newer combination birth control pills (Yaz 28 and Loestrin 24 Fe) that have 24 days of active hormone pills, followed by 4 days of placebo. 24
Storage of Conceptive Pills:
Contraceptive pills should be stored in a dry and cool place. They do not need to be refrigerated. Also, they should be stored properly to avoid accidents of mistaken consumption by children. 25
Guidelines:
In 2000, the American College of Obstetricians and Gynecologists (ACOG) issued practice guidelines regarding hormonal contraception. Also in 2000, the World Health Organization (WHO) published guidelines on medical eligibility for contraceptive
use. The two sets of guidelines are similar.
Cost:
Many of the traditional COCs are cheaper than the new ones and have generic alternatives.
• For a 28-day supply of ethinyl estradiol 20 µg-drospirenone 3 mg or ethinyl estradiol 20 µg-norethindrone 1 mg, the 24/4 extended-cycle regimen costs about $55.
• For a 28-day supply of ethinyl estradiol 20 µg-levonorgestrel 90 µg, the continuous-cycle regimen costs about $50.
• A 91-day supply of ethinyl estradiol 30 µg-levonorgestrel 150 µg (generic equivalent) or ethinyl estradiol 30 µg-levonorgestrel 150 µg with ethinyl estradiol 10 µg in place of placebo costs $150-165.
Brands of various oral contraceptives:
• ella, Plan B, Plan B One-Step and Next Choice are the only dedicated product specifically marketed for emergency contraception.
• Aviane, Cryselle, Enpresse, Jolessa, Lessina, Levora, Lo/Ovral, LoSeasonique, Low-Ogestrel, Lutera, Lybrel, Nordette, Ogestrel, Portia, Quasense, Seasonale, Seasonique, Sronyx and Trivora have been declared safe and effective for use as ECPs by the United States Food and Drug Administration.
• The progestin in Cryselle, Lo/Ovral, Low-Ogestrel and Ogestrel is norgestrel, which contains two isomers, only one of which (levonorgestrel) is bioactive; the amount of norgestrel in each tablet is twice the amount of levonorgestrel.
Conclusions and Recommendations:
For most women who are, like the woman in the vignette, healthy and free of cardiovascular disease and major cardiovascular risk factors, the use of combination estrogen–progestin oral contraceptives is associated with low relative and absolute risks of cardiovascular disease.
Even when the health risks are taken into account, the net health benefit of oral-contraceptive use in these women is great, especially given the effect on the risk of ovarian cancer and its effectiveness in preventing pregnancy. The favorable risk–benefit ratio for healthy women applies to all oral contraceptives containing a low dose of estrogen (less than 50 μg).
Oral contraceptive use should be discontinued immediately in any woman with symptoms suggestive of stroke, myocardial infarction, or venous thrombosis. Oral-contraceptive users should be screened regularly for cervical neoplasia but do not require more frequent screening than nonusers.
Overall, the extended- and continuous cycle oral contraceptive regimens are viable options for women who prefer a decrease in their scheduled withdrawal bleeding or who have coexisting medical conditions and would benefit from less withdrawal bleeding each year
References:
1. Black A, Kives S, Leboeuf M, et al. Canadian consensus guideline on continuous and extended hormonal contraception, 2007. SOGC clinical practice guideline, No. 195, July 2007. J Obstet Gynaecol Can 2007;195:S1-32.
2. Hatcher RA, Zieman M, Cwiak C, et al. A pocket guide to managing contraception. Tiger, GA: Bridging the Gap Foundation, 2005.
3. Duramed Pharmaceuticals. The truth about fewer periods. Available from http://www.fewerperiods.com. Accessed September 7, 2007.
4. Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000;355:922-4.
5. Wiegratz I, Hommel HH, Zimmermann T, Kuhl H. Attitude of German women and gynecologists towards long-cycle treatment with oral contraceptives. Contraception 2004;69:37-42.
6. Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception: an international study on acceptability. Contraception 2003;67:1-8.
7. Andrist LC, Arias RD, Nucatola D, et al. Women's and providers' attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception 2004;70:359-63.
8. Sulak PJ, Buckley T, Kuehl TJ. Attitudes and prescribing preferences of health care professionals in the United States regarding use of extended-cycle oral contraceptives. Contraception 2006;73:41-5.
9. Bayer Pharmaceuticals. Yaz (drospirenone-ethinyl estradiol) package insert. Wayne, NJ; 2007.
10. Warner Chilcott. Loestrin 24 Fe (norethindrone acetate-ethinyl estradiol) package insert. Rockaway, NJ; 2006.
11. Rosenberg MJ, Meyers A, Roy V. Efficacy, cycle control and side effects of low- and lower-dose oral contraceptives: a randomized trial of 20 µg and 35 µg estrogen preparations. Contraception 1999;60:321-9.
12. Nakajima ST, Archer DF, Ellman H. Efficacy and safety of a new 24-day oral contraceptive regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 µg (Loestrin 24 Fe). Contraception 2007;75:16-22.
13. Hatcher RA, Trussell J, Stewart F, Nelson AL, Cates W, Kowal D, eds. Contraceptive technology, 18th ed. New York: Ardent Media, Inc., 2004.
14. Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson- Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol 2005;106:492-501.
15. Anderson FD, Hait H, for the Seasonale-301 Study Group. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003;68:89-96.
16. Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): a 2-year multicenter open-label extension trial. Am J Obstet Gynecol 2006;195:92-6.
17. Killick SR, Fitzgerald C, Davis A. Ovarian activity in women taking an oral contraceptive containing 20 µg ethinyl estradiol and 150 µg desogestrel: effects of low estrogen doses during the hormone-free interval. Am J Obstet Gynecol 1998;179:S18-24.
18. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception 2006;73:229-34.
19. Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle versus cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005;3:CD004695.
20. Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003;101:653-61.
21. Archer DF, Jensen JT, Johnson JV, Borisute H, Grub GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006;74:439-45.
22. Organon Pharmaceuticals. NuvaRing (etonogestrel-ethinyl estradiol) package insert. Roseland, NJ; 2005.
23. Ortho-McNeil Pharmaceuticals. Ortho Evra (norelgestromin- ethinyl estradiol) package insert. Raritan, NJ; 2006.
24. Oddsson K, Leifles-Fischer B, Wiel-Masson D, et al. Superior cycle control with a contraceptive vaginal ring compared with an oral contraceptive containing 30 mcg ethinyl estradiol and 150 mcg levonorgestrel: a randomized trial. Hum Reprod 2005;20:557-62.
25. Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstet Gynecol 2004;104:555-63.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org









