(20th June, 2014); Boehringer Ingelheim and Eli Lilly and Company presented results of two Phase III clinical trials studying the efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in adults with Type 2 Diabetes (T2D) at the American Diabetes Association's 74th Scientific Sessions®. Results showed that empagliflozin in combination with certain other diabetes therapies reduced blood glucose, body weight and blood pressure.




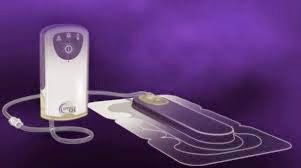 (Business Wire India; 5th June, 2014); Kinetic Concepts, Inc. announced today that a new study, published in the International Wound Journal, showed the Prevena™ Incision Management System used over closed incisions for the first six to seven days reported a significantly lower infection rate than conventional dressings in patients undergoing cardiac surgery via median sternotomy. Post-sternotomy wound infection is a serious, potentially fatal complication of cardiac surgery which often requires additional surgery, and is associated with significantly increased health care costs.
(Business Wire India; 5th June, 2014); Kinetic Concepts, Inc. announced today that a new study, published in the International Wound Journal, showed the Prevena™ Incision Management System used over closed incisions for the first six to seven days reported a significantly lower infection rate than conventional dressings in patients undergoing cardiac surgery via median sternotomy. Post-sternotomy wound infection is a serious, potentially fatal complication of cardiac surgery which often requires additional surgery, and is associated with significantly increased health care costs.





