Q.3.(a)Spin-spin splitting and factors influencing coupling constant
[adsense:336x280:8701650588]
Ans.3. (a)Spin-spin splitting: In the simplest case we expect to see a single peak for each type of proton in a molecule. But consider what happens if a proton that we are looking at (HA) is near another non-equivalent proton (HB). In half of the molecules the HA proton will be adjacent to an HB aligned with the field and in the other half the HA proton will be adjacent to an HB aligned against the field. Thus, half the HA's in the sample will feel a slightly larger magnetic field than they would in the absence of HB and half will feel a slightly smaller magnetic field. Thus, we will observe two absorptions for the HA proton. (Of course we would also observe the same thing for HB.) This splitting of the HA resonance into two peaks is termed "Spin-spin coupling" or "spin-spin splitting" and the distance between the two peaks (in Hz) is called the "coupling constant" (Usually represented by the symbol J). The spin-spin coupling is transmitted through the electrons in the bonds and so depends on the bonding relationship between the two hydrogen’s
Rules for splitting of proton signals:
In general the following set of rules may be found very helpful in predicting or interpreting the splitting of proton signals.
(1). Splitting of a proton signal is caused only by neighbouring or vicinal protons (i.e. Protons on adjacent carbon atoms) which are not equivalent to the proton under consideration. Non-equivalent protons are, of course, those protons which have different chemical shifts. For instance, in the spectrum of CH2Cl-CH2Cl, there is no splitting as the vicinal protons are equivalent to one another.
(2). Splitting of one proton by another on the same carbon very rarely comes across because such protons are generally equivalent each to other.
At the same, the mutual splitting of protons, separated by more than two atoms is also very uncommon. For instance, let us consider the compound 1-bromo-2, 2-dimethylpropane. In this compound there is no splitting of signals as the non-equivalent protons are separated by more than two carbon atoms.
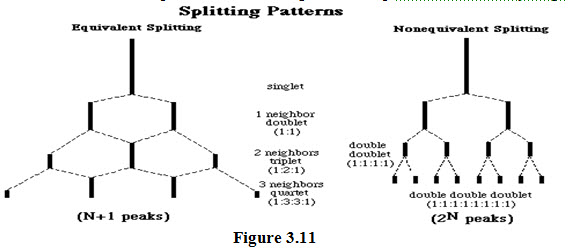
(3). the number of peaks (N) into which a proton signal is split up is equal to one more than the number of vicinal protons (n). That is:-
Thus, The NMR signal due to a proton is split into:
A doublet (with peak intensities 1:1)by one vicinal proton
A triplet (with peak intensities 1:2:1)by two vicinal protons.
A quartet (with peak intensities 1:4:4:1)by three vicinal protons.
A quintet (with peak intensities 1:4:6:4:1) by four vicinal protons and so on.
(4). All the peaks of a given multiplet (i.e., doublet, triplet, quarter, etc.) are not of exactly the same intensity. It has been observed that the inner peaks (i.e., the peak nearer to the other coupled multiplets) are larger than the outer peaks. Thus in case of 1,1,2-tribromoethane (CHBr2CHBr2) the doublets and triplets obtained .
Coupling constant:
The distance between the peaks in a given multiplet in a measure of the magnitude of splitting effect. It is referred to as coupling constant and is denoted by the symbol J. Numerical value of J is expressed in Hz or cps. Unlike the chemical shifts, the values of J are independent of the applied field strength and depend only upon the molecular structure. In general, the size of coupling constant is determined by the number and kind of intervening chemical bonds and the spatial relations between the protons as illustrated below as few cases.
(a) For protons attached to the same carbons atom (i.e., geminal protons), the values of J varies from 0-20 Hz depending upon the bond angle and overall structure of the molecule.
(b) For protons attached to adjacent carbon atoms (i.e., vicinal protons), J varies from 2-18 Hz depending upon the spatial positions and the structure of the molecules as a whole. For example. In case of freely rotating groups, protons with anti- conformation have J= 5-12 Hz while protons with gauche conformation have J =2-4 Hz.
Factors influencing coupling constant
The number of bonds intervening between the coupling nuclei is important, since the coupling is transmitted via the electrons of these bonds. It is a convenient notation to indicate this number as a superscript to the symbol for the coupling constant. Thus, direct coupling (coupling of a proton with an attached carbon-13 nucleus, 13C-1H) would be a one bond coupling, 1J; the coupling between protons on a CH2group would be symbolized by 2J; that between protons on adjacent carbons as 3J; and so on.
Geminal coupling, involving protons on –CH2- groups, is strong, 2J being typically 10-18 Hz, but it will only be observed where the gem protons have different chemical shift positions.
Vicinal coupling(three bonds separating the protons) varies from 3J= 0 to 12 Hz in rigid systems, but in freely rotating carbon chains (alkyl groups) it is usually around 8 Hz.
Long-range couplingin alkane systems (extending over more than three bonds- i.e., 4J and longer) is usually vanishingly small, but is observed within rigid systems.Trans coupling in alkene groups (3J, 11-19 Hz) is stronger than cis coupling (J, -14 Hz).
Aromatic couplingdepends on whether the coupling protons are Ortho, Meta or Para to each other, and in simple cases the coupling constant is definitive in deciding the orientation; thus 3Jortho, 7-10 Hz; 4Jmeta, 2-3 Hz; 5Jpara, 0-1 Hz.
Allylic coupling, as in allyl chloride, is the most likely four-bond coupling to be met in nonaromatic molecules and is very small (4J, 0-2 Hz).
Other magnetic nucleipresent in the molecule (14N, 19F, etc.) may increase the complexity of proton spectra, while substitution of deuterium for hydrogen may lead to simplification.
Factors influencing Geminal Coupling
Adjacent electronegative elements - The electronegativity of an attached substituent alters the values of gem coupling, but not always predictably. In groups such as –CH2-X the gem coupling will range from 12 Hz to 9 Hz as the electronegativity of X is increased.
The magnitude of Jgem also varies with the C-Ĉ̂-C bond angle, being of greatest magnitude (10-14 Hz) in the strain-free cyclohexanes and cyclopentanes. With increasing angular strain the value of Jgem drops, being 8-14 Hz in cyclobutanes and 4-9 Hz in cyclopropanes.
Adjacent π-bonds -The 2J coupling constants for a simple hydrocarbon Such as methane is -12 Hz. When the C-H bond is able to overlap with a neighbouring π bond, the coupling constant is made more negative, in other words effectively larger. The effect is greater when the hyper conjugation is with the π-bond of a carbonyl group than when it is simply with a C=C double bond. Thus in acetone, the coupling constant is -14.9 Hz and in toluene it is -14.3 Hz.
Angle strain -An increase in the H-C-H angle makes 2J more positive, in other words smaller. This effect is most noticeable in the methylene groups of alkenes, where the angle has reached near to 120° and the coupling constant is close to zero. This coupling is very dependent upon the nature of substituents at the other end of the π-bond, electronegative elements, e.g. fluorine, making them more negative and electropositive elements, e.g. lithium, actually making the coupling positive and quite large
Factors influencing Vicinal Coupling
The dihedral coupling -Coupling is mediated by the interaction of orbitals within the bonding framework. It is therefore dependent upon overlap, and hence upon the dihedral angle. The relationship between the dihedral angle and the vicinal coupling constant 3J is given theoretically by the Karplus equations:
3Jab = J0cos2ϕ – 0.28 (0° ≤ ϕ ≤ 90°)
3Jab = J180cos2ϕ – 0.28 (90° ≤ ϕ ≤ 180°)
Where J0 and J180 are constants which depend upon the substituents on the carbon atoms and ϕ is the dihedral angle.
The presence of electronegative or electropositive elements Electronegative elements directly attached to the same carbon atom as one of the vicinally coupled protons reduce the coupling constant, and electropositive elements raise it. For freely rotating chains, the effect is small (1-3).
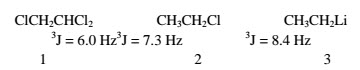
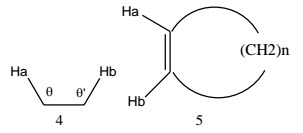
Angle strain -In the fragment 4, 3J decreases as θ and θ’ increase, This effect is noticeable in the cis coupling constants between the olefinic protons in cyclic alkenes (5); as the ring size changes, the coupling constant changes. It is therefore possible to tell in many cases into what size ring a double bond is incorporated.
Bond length dependence - Aromatic carbon-carbon bonds have bond lengths intermediate between normal single and double bonds. In consequence, ortho coupling constants are typically about 8 Hz, rather lower than cisolefinic coupling constants in cyclohexenes (8.8-10.5 Hz).









