Q.3. (b) Simplification of complex proton NMR spectrum
[adsense:336x280:8701650588]
Ans.3. (b)Simplification of proton NMR spectra
Introduction: The complete analysis of a compound is frequently made difficult, when signals overlap and as a result, useful information is often buried due to complexity of the spectrum. In a spectrum several signals may overlap as is the case, e.g., of closely related methylene groups in a molecule. In such a situation an intense, broad and compulsory unresolved signal, termed the methylene envelope may appearsbetween δ 1-2. Another cause of complexity is where a coupling constant is comparable with the chemical shift different between the coupled protons. For these problems following techniques may use to solve them.
NMR spectra may be complex with following complicating factors
1. Overlap: - Overlap is a serious problem in NMR spectroscopy. Integration (which is used to determine the ratio of different kinds of magnetically nonequivalent protons in a sample by measuring the relative intensities of the associated peaks) is of greatest helf in demonstrating the presence of overlap. Perhaps the great difficulty encountered in the application of electronic integration is overlap or resonance peaks, either partial or complete. If facilities for operating a spectrometer at considerably different frequency are available and there is no overlap of peaks on centre, it may be impossible to obtain a spectrum which allows the overlap to be disintegrated. Commercial NMR spectrometers operating at higher and higher frequency are now available, these instruments increase the possibility of the shift and this reduce overlap.
2.Exchange: Complications in the spectrum also arise when two or molecular species are present and both of them carry labile protons which may exchange with each other. If the rate is very slow, then the peak of each type of labile proton will be seen at the usual position and of almost normal appearance. But as the rate of exchange increases, the peak broaden still further and moves towards each other and when the rate became greater than the chemical shift between them, the peaks coalesce. Further increase in the rate only sharpness up the line. Spin spin coupling may also be destroyed by exchange.
3. Hydrogen bonding:-if the structure of the sample molecule is such that strong hydrogen bonding takes place, then the proton involved will have a chemical shift to a much lower field than would otherwise be expected. The same effect of a low field shift may also occur, but somewhat diminished, when hydrogen bonding is molecular as in acids and alcohols.
4. Solvent effects: - The presence of solvent also affects the NMR spectrum. The effect cannot be eliminated. However, it may be standardized. Solvents which are particularly suspected include acetone, benzene, and acetonitrile carbon disulphide usually has little effect on proton spectra, but may strongly influence those of fluorine. The behavior of cyclohexane, carbon tetrachloride and tetra-methylsilane are generally satisfactory
Spectroscopy tricks used in the complex proton NMR spectra:
There are number of tricks which may be used to assist in the interpretation of spectrum For example (1) Active hydrogen atoms of hydroxyl and amine groups may be removed by shaking the solution of the compound in deuterated chloroform with heavy water and then separating the aqueous layer. The signal due to the hydroxyl and amino groups would have disappeared when the spectrum was recorded again. Hence any coupling between the hydroxyl and amino groups and other hydrogen atoms would also have been removed as a result of deuteration of these groups. (2) Double resonance is another important technique which assists in sorting out the spectra. Whether two groups of resonance signals are interacting by spin coupling or not may be ascertained by applying a strong radio frequency irradiation at the centre of the first signal (while observing the second signal). The two groups will be interacting if the second signal is collapsed to a singlet or is simplified.
Some other approaches used in simplify a complex spectrum:-
1. Magic angle spinning and cross polarization: it is also a technique of simplify a complex spectra and the procedure consist of four basic timed sequences of rf pulses. The four part procedure is
a.Polarizing the 1H spin system by applying a 900rf pulse at the 1H resonance frequency.
b. Spin locking in the rotating frame by applying a 900 phase shift to the foregoing field
c. Establishing 13C -1H contact by applying a rf field at the 13C resonance frequency
d. Observing the 13C free induction decay while the 1H field is maintained for decoupling
The entire sequence is repeated many times until a suitable signal-to-noise ratio for 13C is achieved.
1. Use of Shift Reagents:
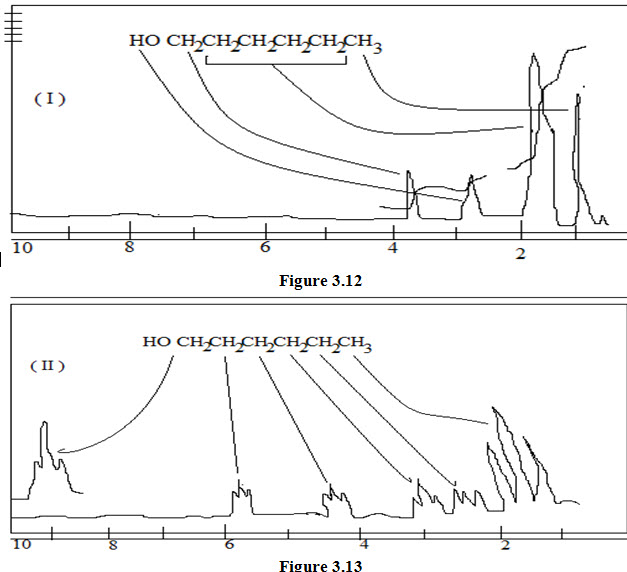
Shift reagents; provide a useful technique for spreading out 1H NMR absorption patterns which normally overlap, without increasing the strength of the applied magnetic field. The 1H NMR spectrum of n-hexanol is reproduced in fig 1.9, 100 MHz which is the normal record. In this spectrum the high field triplet is distorted which represents the absorption of a methyl group adjacent to a –CH2- group. The low field broad multiplet (presumably not first order) is due to the methylene group adjacent to the hydroxyl group. The protons of the remaining methylene groups areall buried in the methylene envelope between δ 1.2 & 1.8. When the same spectrum is recorded (fig. 1.10) after the addition of a soluble europium (III) complex, Eu (DMP)3 , i.e., the shift-reagent, the spectrum is spread out over a wider range of frequencies so that it is now simplified almost to first order. In the spectrum (fig 1.10) the OH absorption signal is shifted too far to be observed. In complexes of this type, the lanthanide ion can increase its co-ordination by bonding interaction with lone pair electrons of groups like NH2, OH, C=O, -O-, COOR, CN. The magnetic field associated with the metal ion, which is a paramagnetic moiety, causes marked changes in the observed shifts of the protons in the substrate- thus the name shift reagent.
2. High Field Strengths: It has been seen that chemical shift in hertz is directly proportional to the applied field, but the chemical shift value δ is independent of field strength. The magnitude of coupling constants J is not dependent on the strength of the applied field but their appearance in the spectrum does change as the magnetic field changes. One cause of complexity in the 1H NMR spectra relates to the situation where the chemical shift difference between the coupled protons becomes comparable to the coupling constant. At a high field all spectra reduce to the simple first order type since now ∆√ ˃˃ J. thus, e.g., an AB coupling pattern from a 60 MHz spectrum tends to become an AX type from, e.g. a 300 MHz spectrum, and the signals become easily recognizable, and otherwise unresolved signals spread out. Moreover, too closely spaced signals are also resolved better at larger field strengths.
3.Spin Decoupling (Double resonance, Double irradiation): Coupling between neighbouring nuclei gives splitting patterns and their analysis is useful for structure determination of compounds. In some situations, however these patterns are so complex that simplification of the spectra is desirable and spin decoupling provides one such techniques. Example of the 1H NMR spectrum of ethanol serves as good example where the spin spin splitting of the methyl signal by the methylene protons is due to the three possible spins states, which the two methylene protons can adopt. If one irradiates strongly the methylene protons with an additional radio frequency at their resonance position, the methylene protons will get induced to change their spin states very rapidly. The methyl protons will therefore only “see an average CH2 state” and coupling will disappear, consequently the methylene absorption will be eliminated and the methyl signal will be recorded at its usual frequency only as a singlet.
4. Deuterium Labelling and Exchange: Deuterium 2H or D, the heavy isotope of hydrogen, has been used extensively in 1H NMR spectroscopy for two reasons. First, it is easily introduced into a molecule. Second, the presence of deuterium in a molecule is not detected in a 1H NMR spectrum. Deuterium has a much small magnetic dipole moment than hydrogen, and therefore it absorbs at different field strengths. Although it broadens the peak of a neighbouring proton, it does not split it because it couples only slightly with a proton. When deuterium replaces the methyl hydrogen’s in, for e.g., ethyl bromide. Note that the multiplicity of the –CH2- peaks changes from a quartet (3+1 peaks) to a triplet (2+1 peaks) to a doublet (1+1 peaks) and finally to a singlet as the hydrogen’s on the –CH3 group are replaced by D. In Br-CH2-CD3 no splitting occurs between the remaining hydrogen’s and the neighbouring methyl, which is now completely deuterated. Carboxylic acid and aldehyde protons appear between δ 12 and 9. Acidic compounds can be differentiated from aldehyde because protons attached to a heteroatom exchange readily with D2O.









