About Authors:
 RAVISANKAR.M*1, SUBASINI.U2, ANAND THANGADHURAI.S3, KARTHIKEYAN.S4, CHANDRA SEKAR.E.5
RAVISANKAR.M*1, SUBASINI.U2, ANAND THANGADHURAI.S3, KARTHIKEYAN.S4, CHANDRA SEKAR.E.5
1.Swamy Vivekanandha College of pharmacy, dept of pharmaceutical analysis. Thiruchengode
2.Swamy Vivekanandha College of pharmacy, dept of pharmacognosy. Thiruchengode
3.Swamy Vivekanandha College of pharmacy, dept of pharmaceutical analysis. Thiruchengode
4.Kausikh therapeutics and private limited.chennai
5.Swamy Vivekanandha College of pharmacy, dept of pharmaceutical analysis. Thiruchengode
ABSTRACT
An isocratic reverse phase high performance liquid chromatographic method for estimation of montelukast sodium and levocetirizine in bulk dosage and in marketed formulations has been devised and validated. The chromatographic separation achieved on shodex c18-4E column (5µm, 250 mm x 4.6 mm) and acetonitrile: methanol: ammonium acetate buffer (PH- 5.5) in the ratio of 25:55:20 v/v. The flow rate was 1.0 ml/min and the UV detection was identified at 225nm.The retention times for montelukast sodium and levocetirizine was found to be 5.15 min and 3.12 min respectively. The linearity of montelukast sodium and levocetirizine is 10 -50µg/ml with the correlation co efficient 0.99 respectively. The validation parameters such as accuracy, precision, LOD, LOQ, Robustness, ruggedness were performed as per ICH guidelines. This method can be used for routine analysis of montelukast sodium and levocetirizine in bulk and marketed dosage forms.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1256
INTRODUCTION
Montelukast sodium is a selective orally active leukotriene, receptor antagonist that inhibits the cysteinyl leukotriene CysLT1 receptor. It is used for the treatment of asthma. Chemically it is a [R-(E)]-1-[[[1-[3-[2-(7-chloro-2quinolinyl) ethenyl]phenyl]-3-[2-(1-hydroxy-1-methyl ethyl) phenyl] propyl] thio] methyl] cyclopropaneacetic acid, monosodium salt. Montelukast sodium is a hygroscopic, optically active, white to off white powder Freely soluble in ethanol, methanol and water.
Levocetirizine is a H1 receptor antagonist. It is used to treat allergies and chronic hives that are due to unknown causes. Chemically it is a R-(+)-2-[2-[4-[(4-chlorophenyl) phenyl methyl] piperazin-1-yl] ethoxy] acetic acid .It is a white or almost white powder and freely soluble in water. Figure 1 and 2 shows the structure of montelukast sodium and levocetirizine respectively.
Literature survey shows that the simultaneous estimation of montelukast sodium and Levocetirizine by hplc has been reported[1-5]. The simultaneous estimation of montelukast sodium and levocetirizine by HPTLC also reported [5, 7-8]. UV methods for analysis of montelukast with levocetirizine [10-12] and the stability indicating hplc method for the montelukast sodium in tablets and in human plasma were studied and reported[6].TLC method for the analysis of these drugs in solid dosage form also reported[9]. The aim of the present work to develop the simple, economical, accurate, reliable reverse phase HPLC method for the estimation of montelukast sodium and levocetirizine in bulk and combined dosage form. This method validated by as per ICH guidelines. Suitable statistical tests were performed on validation report.
[adsense:468x15:2204050025]
MATERIALS AND METHODS
Instrumentation and chromatographic conditions
The HPLC system consisted of MERK HITACHI. It consisted of L-7100 model pump and L-7400 UV detector, version 4.1.Shodex C-18-4E column (250 x 4.6mm) 5µm particle.Rheodyne injector with 50 µl of fixed loop. The mobile phase is a mixture of ammonium acetate buffer: methanol: acetonitrile in the proportion of (20:55:25 v/v).Ammonium acetate buffer maintained with PH of 5.5, adjusted by acetic acid. The mobile phase was delivered at the flow rate of 1ml/min the detection was carried out at 225 nm.
Chemicals and reagents
The Reference standards are provided by the kausikh therapeutics and private limited (Chennai).Commercial tablets contains 10mg of montelukast sodium and 5mg of levocetirizine. It is obtained from sun pharma with the brand name montec-lc.All the reagents were used HPLC grade.
Standard preparation
Stock solution of montelukast sodium (80µl) and levocetirizine (40µl) were prepared by 25 mg of montelukast and 25 mg of levocetirizine dissolved in mobile phase and diluted up to 25 ml with volumetric flask. From that concentration of 80 µg/ml montelukast and 40 µg/ml concentration of levocetirizine were prepared.
Sample preparation
Average weight of tablet was calculated. Then the tablet were crushed to fine powder, dose equivalent to 25 mg was transferred to 25 ml volumetric flask, dissolved in a solvent and sonicated for 15 minutes. From this concentration of 80µg/ml montelukast and concentration of 40µg/ml levocetirizine were prepared.
Assay
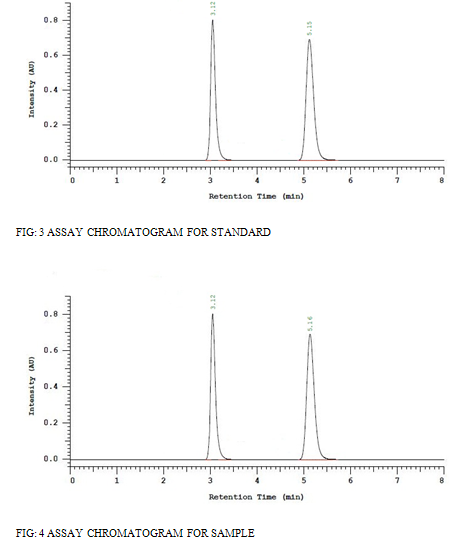
The prepared sample and standard solutions were injected in to the column. Amount present and the percentage purity of the drug was calculated and listed in [Table 8] and Assay chromatograms showed in figure [3] and [4].
Method validation
The developed method has been validated by as per ICH guidelines and the data’s were analyzed by statistically.
Accuracy
To check the accuracy of the proposed method, recovery studies were performed at 80 %, 100%.120% of the test concentration as per ICH guidelines. Three replicates of each concentration were injected in to the column.
Precision
Inter day and intraday precision was performed by six replicates of standard stock solution and area under curve (AUC) were recorded. It is evaluated by calculating standard deviation of resulting data.
Linearity To establish the linearity of the drug five different concentrations of the solutions were prepared from 10µg/ml – 50 µg/ml and injected. The calibration curves were obtained and the correlation co-efficient was calculated by statistically.
LOD and LOQ
The LOD and LOQ determined from slope of the calibration curve.LOD can be determine as signal to noise ratio usually 2:1 or 3:1.LOD can be calculated by the following formula LOD = 3.3 SD/S.
LOQ can be determined as a signal to noise ratio usually 10: 1.LOQ can be calculated by the following formula LOQ = 10 SD/S
Robustness
To investigate the robustness of the developed method, experimental conditions were changed .The flow rate of mobile phase was 1ml/min. To study the effect of flow rate, flow was altered by 0.1 units from 0.9 to 1.1 ml/min. The effect of PH of mobile phase buffer was studied by varying PH ± 0.1 units and other mobile phase components were held constant as stated previously.
Ruggedness
To establish the ruggedness of the method, ruggedness was carried out by using different day with different analysts.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSIONS
The proposed method was validated as per ICH guidelines. Results of accuracy indicate that the method is highly accurate. The percentage of recovery was found to be 99.47 %, 99.52 %, 99.08% for montelukast with % RSD value 0.45, 0.25, 0.46. Respectively. The percentage recovery for levocirizine was 99.76 %, 99.55%, 99.31 % with % RSD value 0.49, 0.51, 0.54 the values are showed in [Table no1].
TABLE 1: RESULTS FOR ACCUARCY
|
|
MONTELUKAST |
LEVOCITIRZINE |
||||
|
AMOUNT PRESENT |
AMOUNT RECOVERED |
% RSD |
AMOUNT PRESENT |
AMOUNT RECOVERED |
% RSD |
|
|
80 % |
8 mg |
7.95 mg |
0.45 |
4 mg |
3.95 mg |
0.49 |
|
100 % |
10 mg |
9.95 mg |
0.25 |
5mg |
4.97 mg |
0.51 |
|
120 % |
12 mg |
11.88 mg |
0.46 |
6 mg |
5.95 mg |
0.54 |
The intraday and intraday precision was investigated. The % RSD for inter day precision was found to be 0.28 and 0.3 for montelukast sodium and levocirizine respectively. The % RSD for intraday precision was 0.85 and 0.11 for montelukast sodium and levocirizine respectively showed in [Table no 2].It reveals that the method shows high precise.
TABLE 2: RESULTS FOR PRECISION
|
PEAK AREA % RSD |
RETENTION TIME % RSD |
|
|
INTER DAY PRECISION (MONTELUKAST) |
0.30 |
0.46 |
|
INTRA DAY PRECISION (MONTELUKAST) |
0.11 |
0.20 |
|
INTER DAY PRECISION (LEVOCETIRIZINE) |
0.28 |
0.90 |
|
INTRA DAY PRECISION (LEVOCETIRIZINE) |
0.85 |
0.45 |
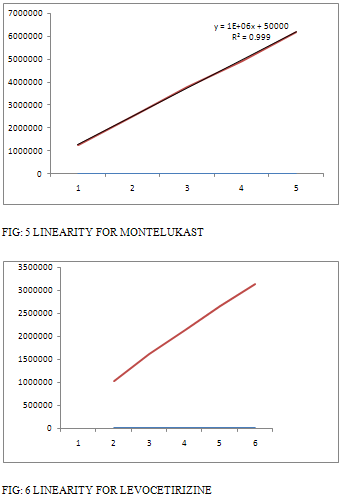
Linearity of the method was determined from the correlation co efficient of calibration curves. The correlation co efficient for montelukast sodium for 0.999 and for levocetirizine 0.999. [Table 3] and Figure [5 – 6].
TABLE 3: RESULTS FOR LINEARITY
|
DRUG |
CONCENTRATION |
SLOPE |
CORRELATION CO-EFFICIENT |
|
MONTELUKAST |
10 – 50 µg/ml |
122999.2
|
0.99 |
|
LEVOCETIRIZINE |
10 – 50 µg/ml |
52764.61 |
0.99 |
The robustness was carried out by changing small variations in flow rate and PH of the mobile phase. By changing the flow rate, obtained % RSD of peak area value was found to be 0.04 and 0.13 for montelukast sodium and levocetirizine respectively. The % RSD for retention time shows 0.77 and 0.95 respectively. [Table 5].The values obtained by changing the Ph of the mobile phase indicated in [Table 4].The% RSD value shows there is no change in method by altering small changes in ph of mobile phase and flow rate.
TABLE 4: RESULTS FOR ROBUSTNESS (VARIATION IN PH)
|
DRUG |
PH |
PEAK AREA |
% RSD |
|
MONTELUKAST
|
5.4 |
10071905 |
0.03 |
|
5.6 |
10081786 |
0.02 |
|
|
LEVOCETIRIZINE |
5.4 |
2868150 |
0.20 |
|
5.6 |
2880347 |
0.04 |
TABLE 5: RESULTS FOR ROBUSTNESS (VARIATION IN FLOW RATE)
|
DRUG |
FLOW RATE ml/min |
PEAK AREA |
% RSD |
|
MONTELUKAST |
0.9 |
10071739 |
0.04 |
|
1.0 |
10079938 |
||
|
1.1 |
10074837 |
||
|
LEVOCETIRIZINE |
0.9 |
2878236 |
0.13 |
|
1.0 |
2879497 |
||
|
1.1 |
2872124 |
Ruggedness were performed with different analysts and reported in [Table 6]. The % RSD value is not more than 2 %.The value indicates it complies with ICH guide lines.
TABLE 6: RESULTS FOR RUGGEDNESS
|
|
RETENTION TIMEOF MONTELUKAST |
PEAK AREA OF MONTELUKAST |
RETENTION TIME OF LEVOCETIRIZINE |
RETENTION TIME OF LEVOCETIRIZINE |
|
ANALYST 1 |
5.14 |
10069827 |
3.11 |
2879796 |
|
ANALYST 2 |
5.15 |
10074148 |
3.12 |
2869998 |
|
ANALYST 3 |
5.14 |
10052739 |
3.14 |
2875694 |
|
ANALYST 4 |
5.15 |
10075638 |
3.13 |
2859649 |
|
ANALYST 5 |
5.16 |
10067758 |
3.12 |
2864647 |
|
% RSD |
0.15 |
0.18 |
0.28 |
0.36 |
LOD is the smallest concentration of the analyte that gives a measurable response. The LOD for the montelukast sodium 0.47 µg/ml and for the levocetirizine 0.69 µg/ml. The LOQ is the smallest concentration of the analyte, which gives response that can be quantified. The LOQ for montelukast sodium was found to be 1.45 µg/ml and for levocetirizine 2.09 µg/ml.[Table 7].
TABLE 7: SUMMARY OF VALIDATION PARAMETERS
|
S.NO |
PARAMETERS |
OBSERVATION |
LIMIT |
PASS/FAIL |
|
|
MON |
LEV |
||||
|
1 |
LINEARITY( µg/ml) |
10-50 |
10-50 |
NO LIMIT |
PASSED |
|
2 |
CORRELATION CO-EFFICIENT |
0.999 |
0.999 |
NLT 0.999 |
PASSED |
|
3 |
ACCURACY 80 %((%RSD) |
0.45 |
0.49 |
NMT 2 |
PASSED |
|
4 |
ACCURACY 100 % (%RSD) |
0.25 |
0.51 |
NMT 2 |
PASSED |
|
5 |
ACCURACY 120% (%RSD) |
0.46 |
0.54 |
NMT 2 |
PASSED |
|
6 |
INTERDAY PRECISION (% RSD FOR PEAK AREA) |
0.30 |
0.28 |
NMT 2 |
PASSED |
|
7 |
INTRADAY PRECISION (% RSD FOR PEAK AREA) |
0.11 |
0.85 |
NMT 2 |
PASSED |
|
8 |
ROBUSTNESS FLOW RATE CHANGE PH CHANGE (% RSD) |
0.04 0.06 |
0.13 0.30 |
NMT 2 NMT 2 |
PASSED PASSED |
|
9 |
LOD µg/ml |
0.47 |
0.69 |
NO LIMIT |
PASSED |
|
10 |
LOQ µg/ml |
1.4 |
2.09 |
NO LIMIT |
PASSED |
|
11 |
RUGGEDNESS % RSD FOR PEAK AREA % RSD FOR RETENTION TIME |
0.18 0.15 |
0.36 0.28 |
NMT 2 NMT 2 |
PASSED PASSED |
TABLE 8: RESULTS FOR ASSAY
|
DRUG |
LABLE CLAIM |
% ASSAY |
AMOUNT PRESENT |
|
MONTELUKAST |
10 mg |
98.81 % |
9.88 mg |
|
LEVOCETIRIZINE |
5 mg |
99.71 % |
4.98 mg |
TABLE 9: SYSTEM SUITABILITY PARAMATERS
|
PARAMETERS/DRUGS |
MONTELUKAST |
LEVOCETIRIZINE |
LIMIT |
|
RETENTION TIME |
5.15 min |
3.12 min |
NO LIMIT |
|
THEORITICAL PLATES |
11224 |
5782 |
NLT 2000 |
|
RESSOLUTION |
12.231 |
NLT 2 |
|
|
TAILING FACTOR |
1.302 |
1.277 |
NLT 2 |
CONCLUSION
The rapid reproducible method RP-HPLC method developed for estimation of montelukast sodium with levocetirizine in bulk dosage forms and in marketed formulation is accuracy, precise, linear, and robust. Satisfactory results were obtained from the validation of the method. The advantages occur in this method, low cost, less running time and high percentage of recovery. So this method can be used for routine analysis of montelukast sodium and levocetirizine in the combined dosage form.
REFERENCES
1. S. Ashokkumar, M.Senthil Raja, .P. Perumal RP-HPLC Method Development and Validation for Simultaneous Estimation of Montelukast Sodium and Levocetirizine Dihydrochloride. International Journal of Pharmaceutical Research 2009 1(4) 8-12
2. Arindam Basu1*, Krishnendu Basak1, Mithun Chakraborty1, Inder Singh Rawat. Simultaneous estimation of levocetirizine and montelukast sodium by RP-HPLC. International Journal of PharmTech Research coden (USA) (2011) ijprif ISSN: 0974-4304 Vol.3, No.1, pp 405-410,
3. B. V.V. Sandeep Kumar*, Mrs. Pratima Mathura, N.Rajesh, D. Narasimha Rao, Panjagala Satyanarayana(2011) analytical method development and validation of levocetirizine hydrochloride and montelukast sodium in combined tablet dosage form by RP- HPLC (2011) IJAPR / July 2011/ Vol. 2 / Issue. 7 / 380 – 396
4. Ambadas R. ROTE* & Vaishali S. NIPHADE (2010). Determination of Montelukast Sodium and Levocetirizine Dihydrochloride in Combined Pharmaceutical Dosage Form by RP-HPLC. Latin American Journal of Pharmacy (2010) (formerly Acta Farmacéutica Bonaerense)Lat. Am. J. Pharm. 29 (6):1020-3
5. Atul S. Rathore, L. Sathiyanarayanan and K.R. Mahadik* Development of Validated HPLC and HPTLC Methods for Simultaneous Determination of Levocetirizine Dihydrochloride and Montelukast Sodium in Bulk Drug and Pharmaceutical Dosage Form. Pharm Anal Acta (2010) 1:106. doi:10.4172/2153- 2435.1000106
6. Ibrahim a. Alsarra development of a stability- indicating hplc method for the determination of montelukast in tablets and human plasma and its applications to pharmacokinetic and stability studies. Saudi pharmaceutical journal(2004), vol. 12, no. 4,
7. Rote* & Vaishali S. Niphadea determination of montelukast sodium and levocetirizine dihydrochloride in combined tablet dosage form by hptlc and first-derivative spectrophotometry. Journal of Liquid Chromatography & Related Technologies (2011) .Volume 34,Issue 3,
8. Sunil R. Dhaneshwar*, Kumudini S. Rasal, Vidhya K. Bhusari, Janaki V Salunkhe and Amruta L. Suryan. Validated HPTLC Method for Simultaneous Estimation of Levocetirizine Hydrochloride and Nimesulide in Formulation. Der Pharmacia Sinica, 2011, 2 (4):117-124
9. Smita Sharma, M. C. Sharma*, D. V. Kohlib, A. D. Sharmac. Development and Validation of TLC-Densitometry Method for Simultaneous Quantification of Montelukast Sodium and Levocetirizine Dihydrochloride Pharmaceutical Solid dosage form. Der Pharmacia Letter, (2010): 2 (1) 489-494
10. Patel Nilam K.1*and Pancholi S. S. Spectrophotometric Determination of Montelukast Sodium and Levocetirizine Dihydrochloride in Tablet Dosage Form by AUC Curve Method. Der Pharma Chemica, (2011), 3 (5): 135-140
11. Lovleen Kumar Garg*1, 2, B. Ravi Kumar1, Dr. Shakil S Sait1, Dr. T. Krishnamurthy1.Determination of montelukast sodium in oral granules dosage forms by simple and accurate UV spectrophotometric methods. International Journal of Pharmaceutical Sciences Review and (2011) Volume 7, Issue 2, Article-012
12. Vishnu P. Choudhari, Anamika N. Kale, Satish A.Polshettiwar, Abhijit S. Sutar, Dhaval M. Patel and Bhanudas S.Kuchekr. Derivative and Absorption Factor Spectrophotometric Estimation of Montelukast Sodium and Levocetirizine Dihydrochloride from Pharmaceutical Formulations. Research Journal of Pharmacy and Technology (2011). Volume 04, Issue 03,
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









