 About Authors:
About Authors:
Rambabu.CH*, V. V. V. S. P. Apparao, Miss. M. Muthulakshmi, V. Ananth.
aPG-Student, Department Of Pharmaceutical Analysis,
KMCH College of Pharmacy,
Kalapatti Road, Coimbatore– 641 048, INDIA.
*ramgepharma@gmail.com
ABSTRACT:
At present, simultaneous determination of drugs in the combination dosage forms has been enjoying renaissance in the field of pharmaceutical analysis. Paracetamol, a classical antipyretic in combination with a novel antispasmodic drug, drotaverine hydrochloride provides a synergistic effect in the treatment of spasms. From the reviewed literature, it was simultaneous uv-spectrophotometric methods have not yet been developed for the determination and quantification of paracetamol and drotaverine hydrochloride. The λmax of paracetamol is 257 nm and that of drotaverine hydrochloride were scanned and found to be 308 nm, 352 nm. Both paracetamol and drotaverine hydrochloride were found to have significant absorbance of the λmax of each other and total absorbance was equal to the sum of the absorbance of paracetamol and drotaverine hydrochloride individually measured. So the present study involves the uv-spectrophotometric method development for the simultaneous determination of paracetamol and drotaverine hydrochloride by using simultaneous equations method. The mean % recoveries from this method were found to be 100.76% and 100.17% for paracetamol and drotaverine hydrochloride respectively proving that the method is accurate.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1471
1. Introduction:
Paracetamol chemically known as N-(4-Hydroxyphenyl) acetanilide is an effective classical antipyretic drug1, drotaverine hydrochloride chemically known as 1-(3, 4-diethoxy benzylidene)-6, 7-diethoxy, 1, 2, 3, 4 tetra hydro isoquinoline is a newer antispasmodic drug2,3. The novel combination both paracetamol and drotaverine hydrochloride has synergistic antispasmodic activity.
From the literature reviewed it was found that only individual determination of paracetamol and drotaverine hydrochloride4 or simultaneous determination of paracetamol along with other drug using UV-Spectrophotometry, HPLC, Electrochemical method etc5,6,7 were available. However, simultaneous determination of paracetamol and drotaverine hydrochloride in combined tablet dosage form by simultaneous equations method using UV-Spectrophotometry was not yet been developed so far which is the present method of interest.
[adsense:468x15:2204050025]
2. Aim and objective:
The combination of Drotaverine and paracetamol are used as an anti spasmodic agent. Paracetamol is an official drug. But Drotaverine is not an official drug. Survey of literature of paracetamol either single or in combination with other drugs revealed several methods based on spectrophotometry and chromatography. But Drotaverine in combination with paracetamol revealed the reverse phase HPLC, first order derivative spectroscopy absorbance ratio method of UV-spectrophotometry. Not a single method has yet been reported for simultaneous determination of both the drugs in combined dosage form.
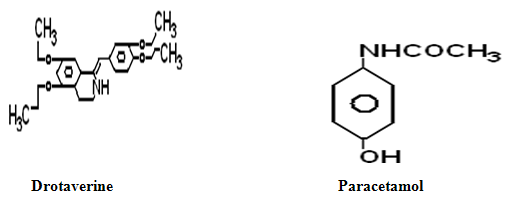
3. Methodology
3.1.Materials:
The raw materials of Paracetamol and drotaverine hydrochloride were obtained as gift samples. The Paracetamol and drotaverine hydrochloride combined tablet dosage form was commercially bought from the local market.
No reagents, other than double distilled water was used.
3.2. Instruments:
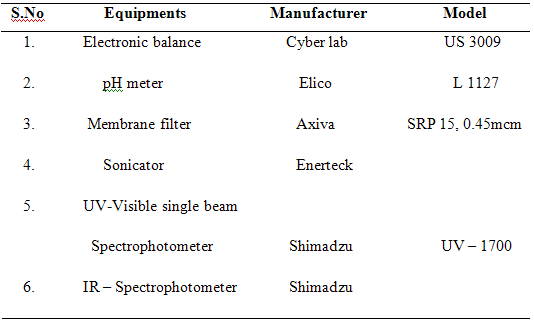
3.3. Identification Tests
i. Melting point - The m.p of Paracetamol was found to be 172oc
The m.p of Drotaverine HCl was found to be 210oc
ii. FT-IR (Fourier transform infrared spectrophotometer):-
IR spectra for standard paracetamol, Drotaverine HCl and mixed standards are interpreted as follows in table 7 (a), 7 (b) & 7 (c).
IR is used for the determination of functional group present in the molecule.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3.4. Quantitative Determination
3.4.1. Procedure for Paracetamol –
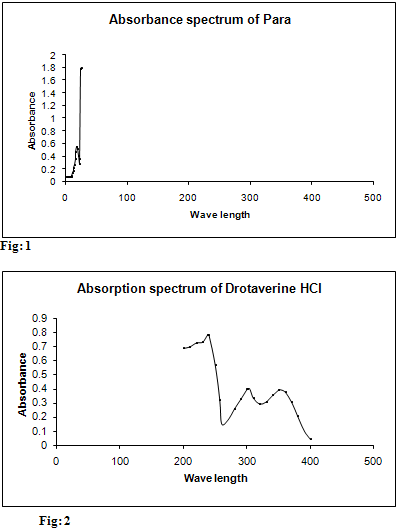
Standard stock solution (A) of paracetamol was prepared by dissolving 10 mg in 2 ml of 0.1M NaOH for increasing solubility and further diluted to 10 ml using water (1000 µg/ml). Then the final concentration for determination was made to 5 µg/ml from Stock solution and scanned in spectrum mode from 200 nm-400 nm. The wave length of maximum absorbance of paracetamol was found out at 257 nm. These are represented in table 1 and Fig.1
3.4.2. Procedure for Drotaverine HCl –
Standard stock solution (B) of drotaverine was prepared by dissolving 10 mg in 2ml of water and further diluted to 10 ml using water (1000 µg/ml). Then the final concentration for determination was made to 5 µg/ml from stock solution and scanned in spectrum mode from 200 nm-400 nm. The maximum absorbance for Drotaverine HCl was and found to be 308 nm, 352 nm. These are represented in table 2 and Fig: 2
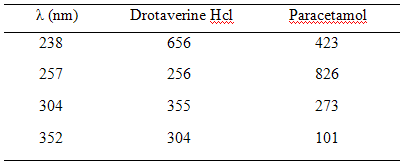
3.4.3. Determination of absorptivities at λmax:
The absorbances of solution of 10 µg/ml concentration of drotaverine HCl and paracetamol were measured at the selected wavelength. The absorbances were divided by the concentration to get absorptivities are presented in Table -3.
A1%1cm = Absorbance/Concentration
3.4.4. Assay in Combined tablet dosage form
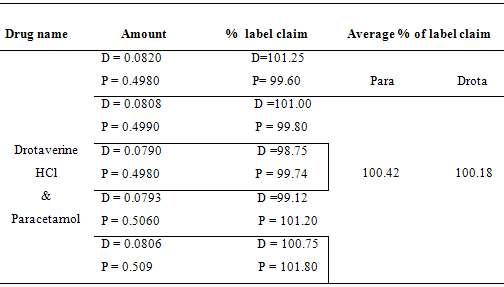
20 tablets were weighed and finely powdered. Weigh the tablet powder equivalent to 50 mg of paracetamol and dissolve it in 100 ml of distilled water (1000 µg/ml). Then the final concentration for determination was made and absorbances were measured at 352 nm, 304 nm, 257 nm and 238 nm. The assay values were tabulated in Table - 6.
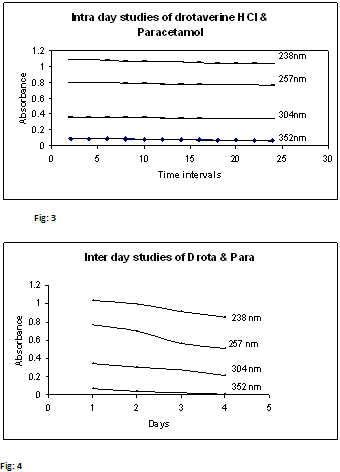
3.5. Intra-day stability studies of Drotaverine HCl & Paracetamol:
10 µg/ml of mixed standard of Drotaverine HCl & Paracetamol was scanned for 24 hrs at intervals of 2 hours separately. To check stability of these solutions, the absorbance values were represented in Table – 4 and Fig.3
3.6. Inter-day stability studies of Drotaverine HCl & Paracetamol:
10 µg/ml of mixed standard of Drotaverine HCl & Paracetamol was scanned at 4 days separately. To check stability of these solutions, the absorbance values were represented in Table – 5 and Fig.4
Table: 1
|
Wave length (nm) |
Absorbance |
|
400 |
0.083 |
|
390 |
0.082 |
|
380 |
0.082 |
|
370 |
0.082 |
|
360 |
0.081 |
|
352 |
0.081 |
|
350 |
0.081 |
|
340 |
0.081 |
|
330 |
0.083 |
|
320 |
0.098 |
|
310 |
0.146 |
|
304 |
0.184 |
|
300 |
0.217 |
|
290 |
0.275 |
|
280 |
0.359 |
|
270 |
0.476 |
|
260 |
0.549 |
|
257* |
0.553 |
|
250 |
0.530 |
|
240 |
0.415 |
|
238 |
0.412 |
|
230 |
0.287 |
|
220 |
0.368 |
|
210 |
1.788 |
|
200 |
1.795 |
Table: 2
|
Wave length (nm) |
Absorbance |
|
400 |
0.042 |
|
390 |
0.103 |
|
380 |
0.204 |
|
370 |
0.307 |
|
360 |
0.378 |
|
352* |
0.395 |
|
350 |
0.393 |
|
340 |
0.353 |
|
330 |
0.308 |
|
320 |
0.294 |
|
310 |
0.334 |
|
304* |
0.402 |
|
300 |
0.396 |
|
290 |
0.326 |
|
280 |
0.257 |
|
270 |
0.181 |
|
260 |
0.153 |
|
257 |
0.319 |
|
250 |
0.566 |
|
240 |
0.780 |
|
238* |
0.783 |
|
230 |
0.731 |
|
220 |
0.725 |
|
210 |
0.698 |
|
200 |
0.687 |
Table: 3 Determination of absorptivities at λmax–
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table:4 - Intra- day Stability Studies of Drotaverine Hcl & Paracetamol :-
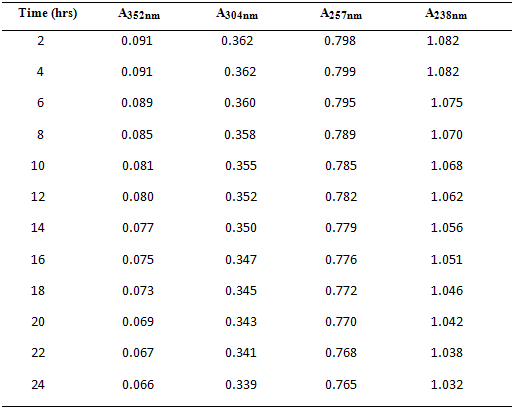
Table:5 - Inter-day stability studies of paracetamol and drotaverine Hcl :-
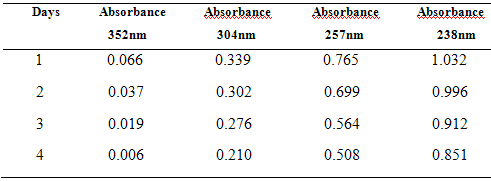
Table:6 – Assay Values in Combined tablet dosage form
Sample ID: Paracetamol
Table 7(a):-
|
S.No. |
Wave number (cm-1) |
Functional group |
|
(1) |
3326 |
O-H stretching |
|
(2) |
3161 |
N-H stretching |
|
(3) |
1654 |
C=O stretching |
|
(4) |
1438 |
C-H bending |
|
(5) |
808 |
Aromatic out of plane bending |
|
(6) |
3036 |
C-H stretching |
Sample ID: Drotaverine HCl:-
Table 7(b):-
|
S.No. |
Wave number(cm-1) |
Functional group |
|
(1) |
2982 |
N-H stretching |
|
(2) |
2342 |
C-H stretching |
|
(3) |
1436 |
C-H bending |
|
(4) |
1039 |
C-O stretching |
|
(5) |
669 |
Aromatic out of plane bending |
|
(6) |
1558 |
N-H bending |
Sample ID: Paracetamol & Drotaverine HCl:-
Table 7(c):-
|
S.No. |
Wave number(cm-1) |
Functional group |
|
(1) |
3325 |
O-H stretching |
|
(2) |
3161 |
N-H stretching |
|
(3) |
2359 |
C-H stretching |
|
(4) |
1561 |
N-H bending |
|
(5) |
1438 |
C-H bending |
|
(6) |
669 |
Aromatic out of plane bending |
|
(7) |
1171 |
C-O stretching |
RESULTS AND DISCUSSION
The analysis of the results shows that the presence of excipients in tablet formulation did not interfere with the final determination of active component. The amount of drug found in formulation is well agreed with label claim. The proposed method can be applied to routine analysis in quality control laboratories.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The results are given in the below table
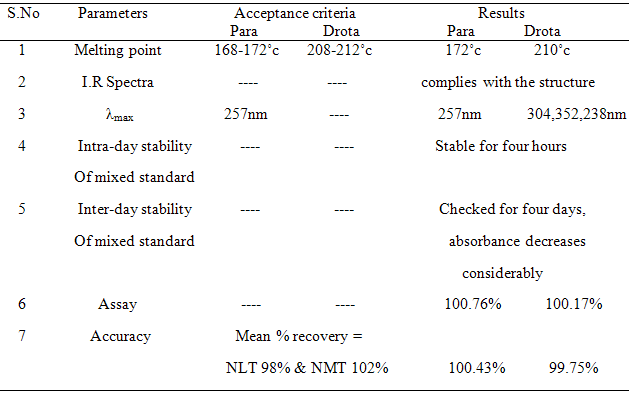
Conclusion –
The proposed simultaneous equation method of UV- Spectrophotometry for the simultaneous determination of Drotaverine HCl and Paracetamol in combined dosage form is simple and accurate.
Hence the present simultaneous equation method of UV – Spectrophotometry is suitable for quality control of raw materials and formulations.
Figure
REFERENCE
1. British Pharmacopoeia, Volume 2, 2004, pp: 1479-1481.
2. sciencedirect.com, wikipedia.
3. R. Sandulescu, S. Mirel and R. Operan, Indian journal of pharmaceutical sciences; Volume 23, Issue 1, 2000, pp: 77-87.
4. G Garg, Swarnalata Saraf, S Saraf; Indian Journal Of Pharmaceutical Sciences; volume 69, Issue 5, 2007, pp; 692-694.
5. S Surana, R Fursule, A Shirkhedkar, S Dari, V Mahajan, P Dahivelkar; Indian Journal of Pharmaceutical sciences; Volume 69, Issue 6, 2007.
6. J Hanaee; Pharmaceutica Acta Helvetiae; Volume 72, Issue 4, Sep. 1997, pp; 239-241.
7. Noordin M.I., Chung L.Y; Drug development and Industrial Pharmacy; Volume 30, Issue 9, 2004, pp; 425-930.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









