 About Authors:
About Authors:
Aditya Dixit1, Gyanendra Kumar Sharma2, Devender Pathak*3
1M. Pharmacy (Pharmaceutical Chemistry)
2Associate Professor (Pharmaceutical chemistry)
3Director, Professor and Head of the Dept. (Pharmaceutical Chemistry)
Rajiv Academy for Pharmacy, Mathura,
Delhi-Mathura Highway, Chhattikara, 281006, India
dev_15@rediffmail.com*, adixit70@gmail.com
ABSTRACT
A traditional but effective method of developing new targets is lead molecule modification, where an existing popular drug from the market is taken as a lead and similar derivatives are synthesized. With the advent of modern techniques conventional methods have lost their gleam but, reactions are still best understood when performed by the conventional techniques. One such endeavor has been made here where indole has been used as a parent moiety and by adding some functional groups some novel indole derivatives were synthesized and screened for their in vitro antitubercular, and anticancer activity. This synthesis of some fresh indole derivatives was done by using various benzaldehyde derivatives and 2-(o-Aminophenyl)indole in acidic condition then with ammonium acetate and isatin in presence of glacial acetic acid.. The structures of newly synthesized compounds were characterized by IR, 1H NMR, MASS and elemental analysis.Compounds 3h & 3i showed good antitubercular activity and 3d, 3e & 3g showed good anticancer compared to the standard drug.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1590
INTRODUCTION
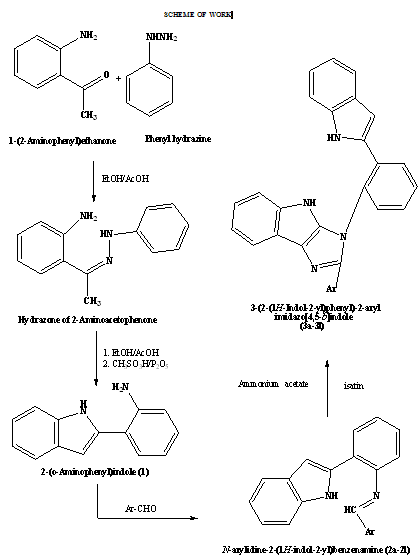
Heterocyclic moieties are a part of most of the drugs existing in the market in one form or the other. Indole is one such moiety which is a part of various anti-inflammatory and analgesic drugs, as it played a crucial role in the development of the COX-2 inhibitors such as Indomethacin, Fendosal, Tenidap etc which occupied a good status in the market for a long time. The indole ring system is a valuable structural moiety having various biologically activities such as, cardioprotective [1], antiviral [2], antibacterial [3], antitumour [4], plant growth regulator [5], antioxidant [6], anti-inflammatory [7], analgesic [8] etc. So looking at the history of Indole and its use, it was thought worthwhile to synthesize some Indole derivatives and evaluate their antitubercular and anticancer activity. In this present work, Phenyl hydrazine on reaction with 2-Aminoacetophenone gave 2-(o-Aminophenyl)indole which on further reaction with various benzaldehyde derivatives gave N-(arylidine)-2-(1H-indol-2-yl)-benzenamines(2a-2l) then these derivatives were reacted with ammonium acetate and isatin to form 3-(2-(1H-indol-2-yl) phenyl)-2-arylimidazo[4,5-b]indoles (3a-3l).
Experimental Section
The purity of all the newly synthesized compounds were checked by TLC on silica gel-protected aluminum sheets (Type 60 F254, Merck) and the spots were detected by exposure to iodine vapors and UV-lamp at λ 254 nm. The melting points were determined in open capillary tubes and were uncorrected. The infrared (FTIR) spectra were recorded on 470-Shimadzu infrared spectrophotometer using the KBr discprepared by pressed pellet technique and expressed in cm-1. 1H NMR spectra were recorded on Bruker DRX-300 using DMSO-d6 as a solvent. The chemical shift was given in δ (ppm) in a downfield manner using tetramethylsilane (TMS) as an internal standard. Splitting patterns were designated as follows: s: singlet; d: doublet; m: multiplet. Elemental analysis was carried on Elemental Vario EL III Carlo Erba 1108 and the values were within ±0.04% of the theoretical values.
[adsense:468x15:2204050025]
Synthetic Procedures
2-(o-Aminophenyl)indole (1)
Step I- Preparation of Phenyl Hydrazone
Phenyl Hydrazine (1.08 gm, 10 mmol) and 2-Aminoacetophenone (1.35 gm, 10 mmol) were mixed in ethanol (20 mL) with few drops of acetic acid and refluxed at 50-60 oC for 10 hours on a water bath. Crystals were filtered and washed with ethanol and then recrystallised with ethanol.
Step II- Cyclization of Phenyl Hydrazone
Methane sulfonic acid (10 mL) was heated to 80oC and then 1.35 gm of P2O5 was added and stirred until it dissolved. Phenyl hydrazone (1 gm) was added slowly and mixture was heated between 80-100oC for half an hour. Reaction mixture was poured on ice and neutralized with NaOH solution. Crude product was filtered and recrystallized with ethanol.
General Procedure
N-(arylidine)-2-(1H-indol-2-yl)-benzenamines(2a-2l)
To an equimolar mixture of 2-(o-Aminophenyl)indole & Ar-CHO, few drops of acetic acid were added with vigorous stirring. The resulting solution was heated under reflux for 3-4 hours. After cooling, the mixture was poured on ice and product was collected after filtration and recrystallised with ethanol.
3-(2-(1H-indol-2-yl) phenyl)-2-arylimidazo[4,5-b]indoles (3a-3l)
Equimolar mixture of intermediate(2a-2l), isatin and ammonium acetate were mixed with ethanol and few drops of acetic acid were added. The mixture was refluxed for 10-14 hours. Then it was cooled to room temp.and poured over crushed ice. Crude product was collected and recrystallised with ethanol.
Derivatives synthesized: (Table-1)
3-(2-(1H-indol-2-yl) phenyl)-2-(o-hydroxyphenyl)imidazo[4,5-b]indole (3a):
IR (KBr, cm-1) ν: 3452.34(O-H, str.), 3338.55 (N-H, str.), 3041.53 (Ar C-H, str.), 1630.59 (Ar C=N, , str.), 1574.09 (C=C, str.), 1350.24 (Ar C-N, str.), 1283.29 (C-O str.). 1H NMR (300 MHz, DMSO-d6 in ppm): δ 5.099 (s, 1H, OH), 6.480(s, 1H, Ar-H), 6.711-6.811 ( d, 2H, Ar-H), 7.005-7.360 (m, 6H, Ar-H), 7.319-7.414 (m, 4H, Ar-H), 7.429-7.624 (m, 4H, Ar-H) 10.029 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 440.4 (100) [M]+, Elemental analysis: Anaylzed for C29H20N4O: Found: C, 79.09%; H, 4.68%; N, 12.45%; O, 3.65%.
3-(2-(1H-indol-2-yl) phenyl)-2-(phenyl)imidazo[4,5-b]indole (3b):
IR (KBr, cm-1) ν: 3334.56 (N-H, str.), 3055.03 (Ar C-H, str.), 1602.74 (C=N, Aromatic, str.), 1563.45 (C=C, str.), 1365.51 (C-N, Aromatic, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): δ 6.412(s, 1H, Ar-H), 7.000-7.329(m, 5H, Ar-H), 7.360-7.512 ( m, 7H, Ar-H), 7.540-7.634 (m, 5H, Ar-H), 10.174 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 424.4 (100) [M]+, Elemental analysis: Analyzed for C29H20N4: Found: C, 82.02%; H, 4.77%; N, 13.25%.
3-(2-(1H-indol-2-yl) phenyl)-2-(2-nitrophenyl)imidazo[4,5-b]indole (3c):
IR (KBr, cm-1) ν: 3359.77 (N-H, str.), 3060.82 (Ar C-H, str.), 1614.31 (C=N, Aromatic, str.), 1582.31 (C=C, str.), 1525.64 (N=O, str.), 1323.77 (C-N, Aromatic, str.), 854.41 (C-N (Nitro)str.); 1H NMR (300 MHz, DMSO-d6 in ppm): δ 6.463(s, 1H, Ar-H), 7.003-7.080 ( d, 3H, Ar-H), 7.191-7.406 (m, 6H, Ar-H), 7.451-7.782 (m, 6H, Ar-H), 8.254 (s, 1H, Ar-H), 10.010 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 469.7 (100) [M]+, Elemental analysis: Analyzed for C29H19N5O2: Found: C, 74.16%; H, 4.06%; N, 14.88%; O, 6.85%.
3-(2-(1H-indol-2-yl) phenyl)-2-(3,4-dimethoxyphenyl)imidazo[4,5-b]indole (3d):
IR (KBr, cm-1) ν: 3346.96 (N-H, str.), 3056.96 (Ar.C-H, str.), 2929.67 (C-H, Aliphatic, str.), 1605.81 (C=N, str.), 1579.32 (C=C, str.), 1326.93 (C-N, str.), 1238.21(C-O-C, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): δ 3.731(s, 6H, (OCH3)2), 6.373 (s, 1H, Ar-H), 6.721-6.938 (m, 3H, Ar-H), 7.010-7.391 (m, 7H, Ar-H), 7.404-7.481 (d, 2H, Ar-H), 7.526-7.664 (d, 3H, Ar-H), 9.754 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 484.5 (100) [M]+, Elemental analysis: Analyzed for C31H24N4O2: Found: C, 76.86%; H, 4.97; N, 11.59%; O, 6.57%.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3-(2-(1H-indol-2-yl) phenyl)-2-(3-nitrophenyl)imidazo[4,5-b]indole (3e):
IR (KBr, cm-1) ν: 3341.24 (N-H, str.), 3027.13 (Ar C-H, str.), 1605.39 (C=N, Aromatic, str.), 1560.45 (C=C, str.), 1510.50 (N=O, str.), 1312.63 (C-N, Aromatic, str.), 850.81 (C-N (Nitro)str.); 1H NMR (300 MHz, DMSO-d6 in ppm): δ 6.488(s, 1H, Ar-H), 7.004-7.224 ( m, 5H, Ar-H), 7.263-7.428 (m, 5H, Ar-H), 7.450-7.835 (m, 5H, Ar-H),, 8.268 (s, 1H, Ar-H), 10.043 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 469.1 (100) [M]+, Elemental analysis: Analyzed for C29H19N5O2: Found: C, 74.20%; H, 4.06%; N, 14.95%; O, 6.78%.
3-(2-(1H-indol-2-yl) phenyl)-2-(4-nitrophenyl)imidazo[4,5-b]indole (3f):
IR (KBr, cm-1) ν: 3339.16 (N-H, str.), 3037.49 (Ar C-H, str.), 1609.67 (C=N, Aromatic, str.), 1556.68 (C=C, str.), 1513.23 (N=O, str.), 1308.17 (C-N, Aromatic, str.), 853.08 (C-N (Nitro)str.); 1H NMR (300 MHz, DMSO-d6 in ppm): d 6.419(s, 1H, Ar-H), 7.099-7.396 ( m, 6H, Ar-H), 7.401-7.610 (m, 6H, Ar-H), 7.653-7.782 (dd, 2H, Ar-H), 8.259-8.267 (dd, 2H, Ar-H), 10.021 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 469.1 (100) [M]+, Elemental analysis: Analyzed for C29H19N5O2 : Found: C, 74.20%; H, 4.06%; N, 14.95%; O, 6.78%.
3-(2-(1H-indol-2-yl) phenyl)-2-(3,4,5-trimethoxyphenyl)imidazo[4,5-b]indole (3g):
IR (KBr, cm-1) ν: 3332.75 (N-H, str.), 3037.10 (Ar.C-H, str.), 2935.12 (C-H, Aliphatic, str.), 1611.56 (C=N, str.), 1585.98 (C=C, str.), 1322.17 (C-N, str.), 1220.73(C-O-C, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): d 3.737(s, 9H, (OCH3)3), 6.415-6.447 (d, 3H, Ar-H), 7.010-7.320 (m, 5H, Ar-H), 7.347-7.501 (m, 4H, Ar-H), 7.522-7.682 (d, 3H, Ar-H), 9.969 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 514.5 (100) [M]+, Elemental analysis: Analyzed for C32H26N4O3: Found: C, 74.68%; H, 5.10%; N, 10.93%; O, 9.30%.
3-(2-(1H-indol-2-yl) phenyl)-2-(4-dimethylaminophenyl)imidazo[4,5-b]indole (3h):
IR (KBr, cm-1) ν: 3347.38 (N-H, str.), 3015.29 (Ar.C-H, str.), 2946.44 (C-H, Aliphatic, str.), 1608.73 (C=N, str.), 1561.49 (C=C, str.), 1247.52 (C-N, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): d2.850(s, 6H, Ar-N(CH3)2), 6.409 (s, 1H, Ar-H), 6.658-6.680 (dd, 2H, Ar-H), 7.002-7.398 (m, 8H, Ar-H), 7..412-7.441 (dd, 2H, Ar-H), 7.506-7.639 (m, 4H, Ar-H), 10.108 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 484 (100) [M]+, Elemental analysis: Analyzed for C31H25N5: Found: C, 79.66%; H, 5.41%; N, 14.95%.
3-(2-(1H-indol-2-yl) phenyl)-2-(4-methoxyphenyl)imidazo[4,5-b]indole(3i):
IR (KBr, cm-1) ν: 3341.48 (N-H, str.), 3019.24 (Ar.C-H, str.), 2942.70 (C-H, Aliphatic, str.), 1617.31 (C=N, str.), 1570.55 (C=C, str.), 1330.83 (C-N, str.), 1206.08(C-O-C, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): d3.739(s, 3H, Ar-OCH3), 6.414 (s, 1H, Ar-H), 6.721-6.891 (dd, 2H, Ar-H), 7.002-7.397 (m, 8H, Ar-H), 7.412-7.526 (dd, 2H, Ar-H), 7.544-7.683 (m, 4H, Ar-H), 10.223 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 467.5 (100) [M]+, Elemental analysis: Analyzed for C30H22N4O: Found: C, 79.30%; H, 4.90%; N, 12.30%; O, 3.50%.
3-(2-(1H-indol-2-yl) phenyl)-2-(2-chlorophenyl)imidazo[4,5-b]indole(3j):
IR (KBr, cm-1) ν: 3340.67 (N-H, str.), 3071.03 (Ar C-H, str.), 1599.54 (C=N, str.), 1567.28 (C=C, str.), 1332.16 (C-N, str.), 813.33 (C-Cl, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): d6.409 (s, 1H, Ar-H), 7.013-7.298 (m, 7H, Ar-H), 7.327-7.444 (m, 5H, Ar-H), 7.479-7.576 (d, 2H, Ar-H), 7.595-7.630 (d, 2H, Ar-H), 10.189 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 458.9 (100) [M]+, Elemental analysis: Analyzed for C29H19ClN4: Found: C, 75.86%; H, 4.14%; Cl, 7.70%; N, 12.23%.
3-(2-(1H-indol-2-yl) phenyl)-2-(3-chlorophenyl)imidazo[4,5-b]indole (3k):
IR (KBr, cm-1) ν: 3341.95 (N-H, str.), 3066.14 (Ar C-H, str.), 1596.78 (C=N, str.), 1564.47 (C=C, str.), 1330.09 (C-N, str.), 811.82 (C-Cl, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): d6.423 (s, 1H, Ar-H), 7.002-7.295 (m, 7H, Ar-H), 7..306-7.522 (m, 6H, Ar-H), 7.549-7.691 (d, 3H, Ar-H), 10.218 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 458.9 (100) [M]+, Elemental analysis: Analyzed for C29H19ClN4: Found: C, 75.86%; H, 4.14%; Cl, 7.70%; N, 12.23%.
3-(2-(1H-indol-2-yl) phenyl)-2-(4-fluorophenyl)imidazo[4,5-b]indole (3l):
IR (KBr, cm-1) ν: .), 3051.17 (Ar C-H, str.), 1609.78 (C=N, str.), 1573.20 (C=C, str.), 1327.44 (C-N, str.), 884.82 (C-F, str.); 1H NMR (300 MHz, DMSO-d6 in ppm): d6.326 (s, 1H, Ar-H), 7.021-7.082 (dd, 2H, Ar-H), 7.123-7.304 (m, 6H, Ar-H), 7.375-7.396 (dd, 2H, Ar-H), 7.428-7.688 (m, 6H, Ar-H), 10.314 (s, 2H, N-H, D2O exchangable); MS (ESI) m/z: 264.2 (100) [M]+, Elemental analysis: Analyzed for C29H19FN4: C, 78.70%; H, 4.26%; N, 12.65%.
Pharmacology
Antitubercular activity [9]
The method followed was Agar dilution method. The principle behind the activity is the inhibition of the number of colony formation. All the samples were tested against the standard antitubercular drug isoniazid. The percentage inhibition by the test compounds was calculated by the formula:
Nt = No. of colonies in sample containing plates.
Nc = No. of colonies in control plate.
Anticancer activity [10]
All the newly synthesized indole derivatives were screened for anticancer activity against MCF 7 (Human breast cancer) cell line by SRB (Sulphorodamine B) assay. The SRB assay possesses a colorimetric end point and is non destructive and indefinitely stable. These practical advances make the SRB assay an appropriate and sensitive assay to measure percent growth inhibition.
RESULTS AND DISCUSSION
All the newly synthesized indole derivatives were synthesized successfully in moderate to good yields. These compounds were identified on the basis of Rf values, solubility in various solvents, melting point range. These compounds were characterized by FTIR, 1H NMR, Mass and elemental analysis. Compounds on 1H NMR analysis, showed the presence of amino group and C-H pyrazole protons between δ 9.265-10.723 ppm and δ 6.701-7.279 ppm respectively, the FTIR analysis showed the presence of characteristic N-H, C=N and C-N peaks within the range 3313-3386 cm-1, 1546-1610 cm-1 and 1317-1388 cm-1 respectively confirming the presence of the indole ring. The N-methylated compounds 3h on 1H NMR analysis showed the presence of protons of the methyl group within the range of δ 2.757-2.863 ppm, the FTIR analysis showed the presence of characteristic methyl C-H peak within the range 2889-2972 cm-1.
Antitubercular activity
Amongst the tested compounds (3a-3l) the compounds 3h (98 %, 12.5 μg/mL) and 3i (98 %,12.5 μg/mL) exhibited good antitubercular activity compared to the standard drug isoniazid (99 %, 12.5 μg/mL). The o-Nitro, o-Chloro, andm-Chloro compounds gave moderate to good activity compared to the other synthesized compounds.
Anticancer activity
Amongst the tested compounds (3c-3g) the compounds 3d, 3e and 3g exhibited good anticancer activity compared to the standard drug Adriamycin. All the derivatives were tested on MCF7 cell line (breast cancer).
CONCLUSION
A series of substituted indole derivatives were synthesized by condensation of 2-(o-Aminophenyl)indole with various benzaldehydes to form N-(Arylidine)-2-(1H indol-2-yl)-benzenamines (2a-2l) which further react with isatin and ammonium acetate to give 3-[2-(1H-Indol-2-yl)phenyl]-2aryl-imidazo[4,5-b]indoles (3a-3l). All of the synthesized compounds were characterized by IR, 1H NMR, MASS and elemental analysis;these synthesized compounds were screened for antitubercular and anticancer activities. The antitubercular activity was performed by agar dilution method and the resulting data suggested that, amongst the tested compounds (3a-3l), the compounds 3h (98 %, 12.5 μg/mL) and 3i (98 %,12.5 μg/mL) exhibited good anti-tubercular activity compared to the standard drug isoniazid (99 %, 12.5 μg/mL). The o-Nitro, o-Chloroandm-Chloro compounds exhibited moderate to good activity compared to the other synthesized compounds.The anticancer activity was performed on MCF7 cell line (breast cancer) and the resulting data suggested that 3d, 3e and 3g compounds exhibited good activity. From the screening data, it may be concluded that the compounds possessing substituents like p-Dimethylamino andp-Methoxy groups enhance the stability of the aryl portion exhibited moderate to good antitubercular activity compared to the standard drug isoniazid. The anticancer activity data suggested that compound 3d, 3e and 3g exhibited promising anticancer activity with GI50 value less than 10.
ACKNOWLEDGEMENT
The authors are also thankful to the management of Rajiv Academy for Pharmacy, Mathura for providing the necessary facilities.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table-1: Physical data of the compounds prepared
|
Com |
Ar |
Mol. Formula |
Rf Value |
Percent |
M.Pt. range |
|
AD 3a |
2-Hydroxy phenyl |
C29H20N4O |
0.68 |
71.11 |
171-173 0C |
|
AD 3b |
Phenyl |
C29H20N4 |
0.81 |
66.23 |
196-198 0C |
|
AD 3c |
2-Nitro phenyl |
C29H19N5O2 |
0.77 |
94.05 |
213-215 0C |
|
AD 3d |
3,4-dimethoxy Phenyl |
C31H24N4O2 |
0.61 |
42.7 |
205-207 0C |
|
AD 3e |
3-Nitro phenyl |
C29H19N5O2 |
0.65 |
88.78 |
223-226 0C |
|
AD 3f |
4-Nitro phenyl |
C29H19N5O2 |
0.56 |
85.21 |
208-210 0C |
|
AD 3g |
3,4,5-tri methoxy phenyl |
C32H26N4O3 |
0.88 |
92.07 |
193-195 0C |
|
AD 3h |
4-Dimethyl amino |
C31H25N5 |
0.66 |
88.28 |
232-235 0C |
|
AD 3i |
4-methoxy phenyl |
C30H22N4O |
0.69 |
90.36 |
215-216 0C |
|
AD 3j |
2-Chloro phenyl |
C29H19N4Cl |
0.76 |
76.45 |
209-210 0C |
|
AD 3k |
3-Chloro phenyl |
C29H19N4Cl |
458 |
0.63 |
222-223 0C |
|
AD 3l |
4-Fluoro phenyl |
C29H19N4F |
442 |
0.67 |
220-221 0C |
Table-2: Results for Anti- ( Human Breast Cancer Cell Line MCF7 ) tubercular activity
|
Compound no. |
Percent inhibition of Mycobacterium tuberculosis in vitro |
|||
|
12.5 μg/mL |
6.25 μg/mL |
3.12 μg/mL |
1.56 μg/mL |
|
|
AD 3a |
70 |
57 |
48 |
35 |
|
AD 3b |
75 |
55 |
59 |
39 |
|
AD 3c |
79 |
43 |
40 |
40 |
|
AD 3d |
74 |
58 |
52 |
47 |
|
AD 3e |
74 |
53 |
45 |
42 |
|
AD 3f |
78 |
65 |
46 |
47 |
|
AD 3g |
78 |
44 |
53 |
44 |
|
AD 3h |
98 |
94 |
55 |
33 |
|
AD 3i |
98 |
89 |
43 |
39 |
|
AD 3j |
85 |
71 |
46 |
33 |
|
AD 3k |
81 |
61 |
57 |
44 |
|
AD 3l |
77 |
60 |
50 |
40 |
|
Isoniazid |
99 |
98 |
99 |
98 |
Table-3: Results for Anticancer activity
|
|
Human Breast Cancer Cell Line MCF7 |
|||
|
|
% Control Growth |
|||
|
Compd. |
Drug Concentrations (μg/mL) |
|||
|
|
10 |
20 |
30 |
40 |
|
Ad 3c |
92.9 |
89.2 |
81.6 |
60.1 |
|
Ad 3d |
12.5 |
-3.0 |
-5.2 |
-27.8 |
|
Ad 3e |
40.4 |
27.4 |
10.3 |
2.1 |
|
Ad 3f |
92.5 |
85.9 |
74.6 |
39.4 |
|
Ad 3g |
43.7 |
19.1 |
10.0 |
2.8 |
|
ADR |
0.3 |
-10.7 |
-33.6 |
-59.7 |
Graphical Abstract
REFERENCES
[1] Abele, E.; Abele, R.; Dzenitis, O.; Lukevics, E. Chemistry of Heterocyclic Compounds, 2003, Chemistry of Heterocyclic Compounds, 39, 3.
[2] Billimoria, A.D.; Cava, M.P. Journal of Organic Chemistry, 1994, 59, 6777-6782.
[3] Biradar, J. S.; Sasidhar, B. S.; Parveen, R. European Journal of Medicinal Chemistry, 2010, 45, 4074.
[4] Carpita, A.; Ribecai, A. Tetrahedron Letters, 2009, 50, 6877.
[5] Cravotto, G.; Demartin, F.; Palmisano, G.; Penoni, A.; Radice, T.; Tollari, S. Journal of Organometallic Chemistry, 2005, 690, 2017.
[6] Dandia, A.; Singh, R.; Singh, D. Indian Journal of chemistry, 2009, 48B, 1001.
[7] Epifano, F.; Genovese, S.; Rosati, O.; Tagliapietra, S.; Pelucchini, C. Tetrahedron Letters, 2011, 52, 568.
[8] Fan, X.; Zhang, Y. Tetrahedron, 2003, 59, 1917.
[9] Garcia, L. C.; Martinez, R. European Journal of Medicinal Chemistry, 2002, 37, 261.
[10] Gawad, H. A.; Mohamed, H. A.; Dawood, K. M.; Badria, F. A. R. Chemical and Pharmaceutical Bulletin, 2010, 58, 1529.
[11] Gholap, S. S.; Wakchaure, P. B.; Pandhare, G. R; Gill, C. H. Indian Journal of Heterocyclic Chemistry, 2009. 18, 270.
[12] Giampieri, M.; Balbi, A.; Mazzei, M.; Colla, P. L.; Ibba, C.; Loddo, R. Antiviral Research, 2009, 83, 179.
[13] Hiroya, K.; Itoh, S.; Sakamoto, T. Tetrahedron, 2005, 61, 10958.
[14] Holla, B. S.; Shashidhara, N. L.; Udupa, K. V.; Poojary, B. Indian Journal of Heterocyclic Chemistry, 2005, 14, 347.
[15] Joseph, M. S.; Totagi, R. S.; Basanagoudkar, L. D. Indian Journal of Chemistry, 2005, 43B, 964.
[16] Liu, H. M.; Xu, W.; Liu, Z. Z. Carbohydrate Research, 2001, 331, 229-232.
[17] Mehta, D. S.; Sikotra, K. H.; Shah, V. H. Indian Journal of Chemistry, 2005, 44, 2594.
[18] Mittapelli, V.; Ray, P. C.; Chauhan, Y. K.; Datta, D. Indian Journal of Chemistry, 2009, 48B, 590.
[19] Nettekoven, M. Tetrahedron Letters, 2000, 41, 8251.
[20] Sachdeva, H. Indian Journal of Heterocyclic Chemistry, 2009, 18, 315.
[21] Subbarayappa, A.; Patoliya, P. U. Indian Journal of Chemistry, 2009, 48B, 545.
[22] Verma, M.; Tripathi, M., Saxena; A. K.; Shanker, K. European Journal of Medicinal Chemistry, 1994, 29, 941.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









