 About Authors
About Authors
M. M. Singhal, Monica Gupta
Department of chemistry,
Delhi Institute of Pharmaceutical science & Research,
New Delhi, India
Affiliation Affiliated from Govt. of NCT Delhi
Abstract :
Carbodithioic acid esters of naphthylmethylamine prepared by replacing the amino function in amino methyl side chain with carbodithioic acid ester group and by adding various S-2-hydroxy propyl ester of dialkyl carbodithioic acid at amino group. Some of these compounds showed better spermicidal activity. The study revealed that incorporation of carbodithioic acid residue directly into naphthylmethylamine leads to compounds with better spermicidal activity and naphathalene-1-yl-methyl-carbodithioic acid-3-dialkyl amino-2-hydroxy-propyl ester has shown better profile than nonoxynol-9. Further, lead optimization may yield a potent spermicide.
[adsense:336x280:8701650588]
Introduction :
The acquired immune deficiency syndrome (AIDS) appears to thrive in the presence of overpopulation, poverty and other sexually transmitted diseases (STDs)1. In spite of the firm resistance of healthy human vagina to HIV infection2, approx 5 million new patients are added annually to near about 40 million living with HIV, half of which are women3. This indicates high prevalence of HIV in heterosexual contacts and increase in number of women with compromised vaginal integrity induced by vaginally applied chemical products. On the other hand, high number of unintended pregnancies4 may also indicate the need for newer, cost-effective, safe and convenient contraceptives.
Vaginal contraceptive products have been available for many years and usually comprise of the membrane surfactant (non-ionic detergent) nonoxynol-9 (N-9) as one of the main ingredients5. However, the major drawback of using N-9 or other surfactants is their detergent-type cytotoxicity toward vaginal cells that has shown to increase the risk of HIV/STI transmission6-8. Besides, N-9 is also known to inactivate Lactobacilli, which further enhances the chances of HIV/STI transmission9. Therefore, developing user controlled non-detergent, topical vaginal spermicides that can provide better protection against pregnancy as well as some very common sexually transmitted pathogens has become an urgent global priority1. Such agents can effectively alleviate the global crisis of unwanted pregnancies and AIDS10.
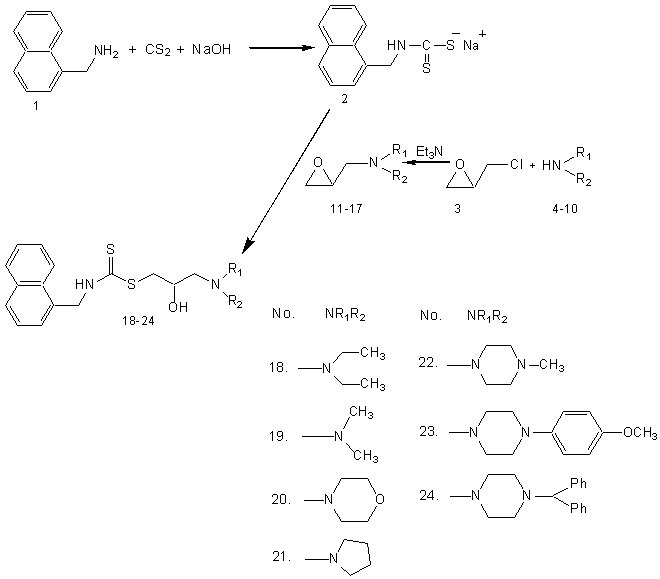
It was reported earlier that propranolol11, a beta-blocking drug, which is known to inhibit human sperm motility and one of the pharmacophore present in propranolol is 1-[3-dialkylamino-propan-2-ol].Previously, no attempts have been made to modify propranololstructure with a view to improve spermicidal activity. The pharmacophores imparting spermicidal activityhave been reported to be dithiocarbamates12, disulfide esters13, thiourea derivatives14, etc.Carbodithioic acid group (II; Fig.: 1) is a valuable pharmacophore that induces diverse biological activities when incorporated in a particular structure1. Therefore, the author were planned to derivatize at amino group of naphthylmethylamine (I; Fig.: 1) moiety to prepare a series of S-[3-dialkylamino propan-2-ol] ester of naphthylmethylaminecarbodithioic acid (III; Fig.: 1). The compounds (2, 18-24) synthesized were screened for spermicidal activity. Nonoxynol-9 was used as reference standard15 for biological evaluation.
Fig: 1

Results and Discussion
Naphathalene-1-yl-methyl-carbodithioic acid-3-dialkylamino-2-hydroxy-propyl esters (18-24) were synthesized by reaction of sodium salt of naphathalene-1-yl-methyl-carbodithioic acid (2, obtained from reaction of naphathalene-1-yl-methylamine with carbon disulphide and aqueous NaOH in the presence ethyl acetate) with epoxy compounds (11-17, obtained from reaction of dialkylamines (4-10) with epichlorohydrin in the presence of triethylamine). The formed compounds were characterized by spectral data.
Eight compounds (2, 18-24) were evaluated in vitro for their spermicidal activity. Five compounds (2, 18, 21, 23 and 24) showed 100% spermicidal activity at concentration of 0.1% (1 mg/ml), in which compounds (18, 21, 23 and 24) also showed 100% spermicidal activity at concentration of 0.05% (0.5 mg/ml). Two compounds (19, 22) showed more than 50% spermicidal activity at concentration of 0.1%, and one compound (20) showed less than 25% spermicidal activity at concentration of 0.1%. N-9 showed spermicidal MEC of 0.05%.
The objective of the study was to evaluate the effect of introduction of carbodithioic acid moiety into naphthalene-1-yl-methyl aminestructure on spermicidal activity. Carbodithioic acid sodium salt of naphthalene-1-yl-methyl amine (2) showed spermicidal activity at concentration of 0.1%(MEC: 0.1%). It has spermicidal action while naphthalene-1-yl-methylamine (1) was found inactive in spermicidal evaluation. In S-(3-substituted amino-2-hydroxy-propyl) esters of naphthalene-1-yl-methylamine carbodithioic acid (18-24), four compounds (18, 21, 23 and 24) were found to be most active at concentration of 0.05% (MEC: 0.05%). Two compounds, dime- thylamino (19) and N-methyl piperazino (22) were found to be moderately active (MEC: 1%) in spermicidal evaluation. Morpholino compound (20) was found to be mild active in spermicidal evaluation. The activity profile of compound 20 suggested that the presence of oxygen in the cyclic amino ring resulted in reduction/loss of activity.
Experimental section
All chemicals and solvents (anhydrous or HPLC grade) were procured from Sigma-Aldrich/Merck India Ltd. Anhydrous sodium sulphate was used as drying agent. Thin layer chromatography was performed on preparative glass plates. Melting points and boiling points were determined in open glass capillaries using paraffin bath and are uncorrected. IR spectra of the compounds were recorded on Nicolet Impact-410 FTIR spectrophotometer. 1H NMR and 13C NMR spectra were recorded on Bruker DPX-300 spectrometer in DMSO and deuterated solvents (CDCl3) with TMS as internal reference (chemical shifts in δparts per million). Mass spectra were recorded on Bruker daltonics, MS Maldi TOF TOF spectrometer.
Structure of all the synthesized compounds was assigned on basis of their analytical and spectral data.
General procedure for the synthesis of naphathalene-1-yl-methyl-carbodithioic acid 2: The compound, naphthylmethylamine (1, 0.01908mol, 2.79ml) was dissolved in 20ml ethyl acetate and cooled in an ice bath with stirring. NaOH (0.02862 mol, 30%) was added drop wise followed by CS2 (0.02289 mol, 1.38ml) and kept stirring for 4 h. Later the reaction mixture was brought to room temperature and stirring was continued for another 10 h.Completion of the reaction was confirmed by TLC in 9.0:1.0 CHCl3:Methanol. After completion of reactionthe ethyl acetate was concentrated under reduced pressure in rotavapor. The residue was taken into hexane and cooled. The separated white-yellowish coloured solid was filtered, recrystallized with ethyl acetate and dried in vacuum desiccater.
General procedure for the synthesis of 1-oxiranylmethyl-diethylamine 11-17: The compound, secondary amine (4-10, 0.1mol) was dissolved in methanol and cooled in an ice bath. Triethylamine (0.09 mol) was added dropwise to the reaction mixture under nitrogen atmosphere followed by addition of epichlorohydrin (3, 0.07 mol) to the reaction mixture and stirring was continued in ice bath for 6-8 h until the reaction was complete (as monitored by TLC). Methanol was evaporated under reduced pressure in rotavapor and the residue was taken into ethyl acetate. The solid triethylamine hydrochloride was filtered and the ethyl acetate layer was dried over anhydrous sodium sulphate. Sodium sulphate was filtered and ethyl acetate was concentrated under reduced pressure in rotavapor. The formed compound was used as it is without further purification.
General procedure for the synthesis of naphathalene-1-yl-methyl-carbodithioic acid-3-dialkyl amino-2-hydroxy-propyl ester 18-24: 1-Oxiranylmethyl-dialkylamine (11-17, 0.01 mol) was dissolved in methanol and naphthalene-1-yl-methyl-carbodithioic acid sodium salt (2, 0.01 mol) was added at room temperaturewith stirring and the stirring was continued for 10-12 h until the reaction was over (as monitored by TLC). Methanol was evaporated under reduced pressure in rotavapor and the residue was taken into ethyl acetate. The ethyl acetate layer was washed and dried over anhydrous sodium sulphate. Sodium sulphate was filtered off and ethyl acetate was evaporated under reduced pressure in rotavapor to get the oily compound. The oily compound was further purified by column chromatography using Hexane:Ethyl acetate (9:1) as the eluent.
Spectral data of some selected compounds
Naphathalene-1-yl-methyl-carbodithioic acid 2:White coloured solid; Yield: 78.85%; M.P.: 228?C; IR (KBr, cm-1): 1597.59 (C=S) and 1275.52 (C-S); 1H NMR (400 MHz, DMSO) δ: 5.11-5.12 (d, 2H, CH2-Ar), 7.40-8.22 (m, 7H, ArH), 3.36-3.41 (s, 1H, NH);13C NMR (100 MHz, CDCl3) δ: 48.45, 124.36-136.03, 215.56;MS (Maldi) m/z: 278.309 [M+Na]+.
Naphathalene-1-yl-methyl-carbodithioic-acid-3-dimethylamino-2-hydroxy-propyl ester 18: Colourless liquid; Yield: 65.03%; B.P.: 152?C; IR (DCM cm-1): 3307.94 (N-H), 1512.05 (C=S), 1275.83 (C-S), 2957.85 & 2929.21 (C-H), 1326.56, 1127.43 (C-O); 1H NMR (400 MHz, CDCl3) δ: 7.47-8.00 (m, 7H, ArH), 5.15-5.16 (d, 2H, CH2-Ar), 4.54 (s, 1H, NH), 4.19-4.25 (m, 1H, CH), 3.44-3.45 (d, 2H, S-CH2), 3.07-3.08 [d, 2H, CH2-N(C2H5)2], 2.72-2.86 (m, 4H, 2×CH2-CH3), 2.30 (s, 1H, OH), 1.36-1.54 (t, 6H, 2×CH3-CH2-); 13C NMR (100 MHz, CDCl3) δ: 14.14, 22.66, 31.59, 45.38, 47.92, 57.32, 58.61, 122.83-133.87, 190.92;MS (Maldi) m/z: 385.615 [M+Na]+.
Naphathalene-1-yl-methyl-carbodithioic-acid-3-dimethylamino-2-hydroxy-propyl ester 19: Colourless liquid; Yield:67.87; B.P.: 145?C; IR (DCM cm-1): 3324.83 (N-H), 2958.54 & 2929.32 (C-H), 1512.11 (C=S), 1275.72 (C-S), 1355.74, 1125.34 (C-O); 1H NMR (400 MHz, CDCl3) δ: 7.48-7.90 (m, 7H, ArH), 5.18-5.19 (d, 2H, CH2-Ar), 4.72 (s, 1H, NH), 4.05-4.26 (m, 1H, CH), 3.43-3.44 (d, 2H, S-CH2), 2.98-3.00 [d, 2H, CH2-N(CH3)2], 2.67 & 2.77 (s, 6H, 2×CH3), 2.36 (s, 1H, OH);13C NMR (100 MHz, CDCl3) δ: 45.37, 47.91, 57.32, 58.61, 122.83-133.87, 190.65-190.92;MS (Maldi) m/z: 357.512 [M+Na]+.
Naphathalene-1-yl-methyl-carbodithioic-acid-2-hydroxy-3-morpholin-4-yl-propyl ester 20: Colourless liquid;Yield: 61.08%; B.P.: 163?C; IR (DCM cm-1): 2959.35, 2930.09 (C-H), 1580.39 (C=S), 1274.95 (C-S), 1380.66, 1123.28 (C-O); 1H NMR (400 MHz, CDCl3) δ: 7.51-8.01 (m, 7H, ArH), 5.16-5.17 (d, 2H, CH2-Ar), 4.65 (s, 1H, NH), 4.12-4.26 (m, 1H, CH), 3.71-3.80 (m, 4H, 2×O-CH2 morpholine), 3.27-3.29 (S-CH2), 2.98-3.00 (d, 2H, 2×-CH-CH2-N), 2.63-2.70 & 2.49-2.56 (t, N-CH2 morpholine), 2.25 (s, 1H, OH);13C NMR (100 MHz, CDCl3) δ: 45.39, 47.92, 57.32, 58.61, 68.13, 122.83-133.87, 191.76;MS (Maldi) m/z: 415.628 [M+Na]+.
Naphathalene-1-yl-methyl-carbodithioic-acid-2-hydroxy-3-pyrrolidin-1-yl-propyl ester 21: Colourless liquid; Yield: 71.65%; B.P.: 156?C; IR (DCM cm-1): 3324.83 (N-H), 2959.53 & 2929.34 (C-H), 1514.15 (C=S), 1275.70 (C-S), 1354.75, 1123.04 (C-O); 1H NMR (400 MHz, CDCl3) δ: 7.39-8.00 (m, 7H, ArH), 5.15-5.16 (d, 2H, CH2-Ar), 4.55 (s, 1H, NH), 4.10-4.24 (m, 1H, CH), 3.40-3.42 (d, 2H, S-CH2), 3.00-3.01 (d, 2H, -CH-CH2-N), 2.79-2.90 (m, 4H, 2×N-CH2 pyrrolidine), 2.25 (s, 1H, OH), 1.93-1.99 (m, 4H, 2×CH2-CH2 pyrrolidine);13C NMR (100 MHz, CDCl3) δ: 10.97, 14.06-14.13, 22.66-23.74, 28.92-31.59, 38.72, 45.35, 47.89, 57.29, 58.58, 68.15, 122.52-133.87, 190.94, 207.00;MS (Maldi) m/z: 383.591 [M+Na]+, 399.592 [M+K]+.
Naphathalene-1-yl-methyl-carbodithioic-acid-2-hydroxy-3-(4-methyl-piperazine-1-yl)-propyl ester 22: Colourless liquid; Yield: 59.07%; B.P.: 158?C; IR (DCM cm-1): 2926.98 (C-H), 1447.20 (C=S), 1275.50 (C-S), 1122.93 (C-O); 1H NMR (400 MHz, CDCl3) δ: 7.50-8.00 (m, 7H, ArH), 5.21-5.22 (d, 2H, -CH2-Ar), 4.55 (s, 1H, NH), 4.02-4.11 (m, 1H, CH), 3.39-3.4 (d, 2H, S-CH2), 3.07-3.09 (d, 2H, -CH-CH2-N), 2.79-2.90 (m, 4H, 2×N-CH2 piperazine), 2.62-2.68 (m, 4H, 2×N-CH2 piperazine), 2.47 (s, 3H, N-CH3), 2.25 (s, 1H, OH);13C NMR (100 MHz, CDCl3) δ: 41.82, 47.92, 51.22, 57.32, 59.34, 123.47-129.47, 192.66;MS (Maldi) m/z: 412.443 [M+Na]+.
Naphathalene-1-yl-methyl-carbodithioic-acid-2-hydroxy-3-[4-(4-methoxy-phenyl)-piperazine-1-yl]-propyl ester 23:Colourless liquid;Yield: 68.34%; B.P.: 172?C; IR (DCM cm-1): 3376.81 (N-H), 2918.04 (C-H), 1512.23 (C=S), 1264.87 (C-S), 1324.35, 1194.28 (C-O); 1H NMR (400 MHz, CDCl3) δ: 7.42-8.01 (m, 1H, 2×ArH), 5.15-5.17 (d, 2H, -CH2-Ar), 4.24-4.29 (m, 1H, CH), 4.15 (s, 3H, OCH3), 3.81-3.84 [t, 4H, 2×CH2-N(piperazine)-Ar], 3.50-3.51 (d, 2H, S-CH2), 3.07-3.11 (t, 4H, 2×N-CH2 piperazine), 2.97-2.98 (d, 2H, -CH-CH2-N), 2.77 (s, 1H, OH), 1.27-1.28 (s, 1H, NH);13C NMR (100 MHz, CDCl3) δ: 14.14, 22.70, 29.37, 29.67, 29.71, 31.93, 45.37, 47.92, 57.32, 58.61, 122.84-133.87, 190.92;MS (Maldi) m/z: 504.283 [M+Na]+.
Naphathalene-1-yl-methyl-carbodithioic-acid-3-(4-benzhydril-piperazin-1-yl)-2-hydroxy-propyl ester 24: Colourless liquid; Yield: 65.67%; B.P.: 179?C; IR (DCM cm-1): 3443.56 (N-H), 2960.08 & 2930.68 (C-H), 1580.41 (C=S) 1287.07 (C-S), 1379.15, 1123.63 (C-O); 1H NMR (400 MHz, CDCl3) δ: 7.12-7.92 (m, 17H, 3×ArH), 5.51 [s, 1H, CH(Ar)2], 5.22-5.23 (d, 2H, CH2-Ar), 4.55 (s, 1H, NH), 4.08-4.21 (m, 1H, CH), 3.39-3.41 (d, 2H, S-CH2), 2.97-2.99 (d, 2H, -CH-CH2-N), 2.76-2.82 (t, 4H, 2×N-CH2 piperazine), 2.59-2.67 (t, 4H, 2×N-CH2 piperazine), 2.22 (s, 1H, OH);13C NMR (100 MHz, CDCl3) δ: 10.96, 13.74, 14.07, 19.16, 22.58-22.99, 23.73, 25.49, 27.71, 28.92, 29.37-30.94, 31.93, 38.70, 65.56, 68.13, 71.79, 128.80-132.43, 167.68-167.76; MS (Maldi) m/z: 564.734 [M+Na]+, 581.002 [M+Na]+.
Spermicidal activity
The test compounds were dissolved in a minimum volume of DMSO and diluted with physiological saline (0.85% NaCl in distilled water) to make a 1.0% (10 mg/ml) solution. The solutions were further diluted serially with saline. A spermicidal test was performed with each dilution starting from 1.0% until the minimum effective concentration (MEC) was arrived.
For this purpose 0.05 ml of liquefied human semen was added to 0.25 ml of test solution and vortexed for 10 s. A drop of the mixture was immediately placed on a microscope slide, covered with a cover glass and immediately examined under a phase contrast microscope in five fields of vision. The results were scored positive if 100% spermatozoa became immotile in 20 s. The MEC was determined in three individual semen samples from different donors. The minimum concentration of compound capable of killing 100% sperm in about 20 s in ‘‘all’’ the semen samples was denoted as MEC. The percentage of immotile spermatozoa was recorded in Table 1.
Table: I
Compound No. % of immotile spermatozoa at concentration (w/v)
1% 0.10% 0.05%
N-9 100% 100% 100%
1 ≤50% Nil Nil
2 100% 100% ≥50%
18 100% 100% 100%
19 100% ≥50% Nil
20 ≤50% ≤25% Nil
21 100% 100% 100%
22 100% ≥50% Nil
23 100% 100% 100%
24 100% 100% 100%
[adsense:468x15:2204050025]
Conclusion
The study demonstrated that incorporation of Carbodithioic acid residue directly into naphathalenemethyl aminestructure leads to compounds with better profile than nonoxynol-9. Further, lead optimization may result into a potent spermicide. However, nonoxynol-9 use is associated with surfactant-type of cytotoxicity that may increase susceptibility to HIV and STD infections, and therefore, the non-surfactant structures 18, 21, 23 and 24 (with more potent spermicidal activity) may serve as safer options in such cases.
Acknowledgement
M. M. Singhal is thankful to Mr. Abhinandan Danodia & Mr. Rajiv Ranjan Jha for their extreme help in research work.
References
1 Kiran Kumar S T V S, Kumar Lalit, Sharma V L, Jain Ashish, Jain Rajeev K, Jagdamba P, Maikhuri, Kumar Manish, Shukla Praveen K, Gupta Gopal, Carbodithioic acid esters of fluoxetine, a novel class of dual-function spermicides, Eu J Med Chem, 43, 2008, 2247-2256.
2 Cone R A, Hone T, Wong X, Abusuwwa R, Anderson D J, Moench T R, BMC Infect Dis, 6, 2006, 90-105.
3 Lederman M M, Offord R E, Hartley O, Nat Rev Immunol, 6, 2006, 371-382.
4 Gamble S B, Strauss L T, Parker W Y, Cook D A, Zane S B, Hamdan S, Centers for Disease Control and Prevention (CDC), Abortion surveillance, United States, 2005. MMWR, Surveill Summ, 57, 2008, 1-32.
5 Zaneweld L J D, Walter D P, Anderson R A, Chany I I, Rencher W F, Feathergil K, Xui Diao X, Doncel G F, Herold B, Cooper M, Biol Reprod, 66, 2002, 886.
6 Stephenson, J JAMA, 284, 2000, 949.
7 Roddy R E, Zekeng L, Ryan K A, Tamoufe U, Tweedy K G, JAMA, 287, 2002, 1117.
8 Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongra- ngson P, Mukenge-Tshibaka L, Ettiegne-Traore V, Uaheowitchai C, Karim S S, Masse B, Perriens J, Laga M, Lancet, 360, 2002, 971.
9 Martin H L, Richardson B A, Nyange P M, Lavreys L, Hillier S L, Chohan B, Mandaliya K, Ndinya-Achola J D, Bwayo J, Kreiss J J, Infect. Disease, 180, 1999, 1863.
10 C. Mauck G F, Doncel, Curr, Infect Dis Rep, 3, 2001, 561-568.
11 Pearson M, Ridgway C J, Johnston A, Vadukul J, Adv Contracept, 1, 1985, 103-108.
12 Tripathi R P, Khan A R, Setty B S, Bhaduri A P, Acta Pharm, 46, 1996, 169–176.
13 Dwivedi A K, Sharma V L, Kumaria N, Kiran Kumar S T V S, Srivastava P K, Ansari H, Maikhuri J P, Gupta G, Dhar J D, Roy R, Joshi B S, Shukla P K, Kumar M, Singh S, Bioorg Med Chem, 15, 2007, 6642–6648.
14 D’Cruz O J, Venkatachalam T K, Uckun F M, Biol Reprod, 63, 2000, 196–205.
15 Kiran Kumar S T V S, Sharma V L, Tiwari P, Singh D, Maikhuri J P, Gupta G, Singh M M, Bioorg Med Chem, 16, 2006, 2509-2512.
Scheme-1:
Abbreviation
Ar Aromatic
B.P. Boiling point
CS2 Carbon disulphide
CHCl3 Chloroform
CDCl3 Deutrated chloroform
Chrom. Chromatography
d Doublet
DMSO Dimethyl sulphoxide
DCM Dichloromethane
Et3N Triethylamine
eq. Equilents
HIV Human immunodeficiency virus
h Hours
M.P. Melting point
m Multiplets
MEC Minimum effective concentration
Ph Phenyl
PPM Parts per million
RBF Round bottom flask
STI Sexually transmitted infection
STD Sexually transmitted disease
s Singlet
t Triplet
TLC Thin Layer Chromatography
T.M.S. Tetramethylsilane
δ Chemical shift
Reference ID: PHARMATUTOR-ART-1004









