About Authors:
About Authors:
Pritesh R. Patel*, Priyank Patel, Jagath Pillai, Nilesh Darji, Bhagirath Patel
Department of Pharmaceutical Chemistry,
Sat Kaival College of Pharmacy, Sarsa,
Ta & Dist: Anand (Gujarat), India-388365
*prit.pharma@gmail.com
ABSTRACT
As part of ongoing studies in developing new antimicrobials, a novel series of 4-acetamido-N-(substituted 1, 3-benzothiazol-2-yl) benzenesulphonamide and N-(substituted 1, 3-benzothiazol-2-yl)-4-(substituted aryl diazenyl) benzenesulphonamide were synthesized in order to determine their antibacterial and antifungal activity. The synthesized compounds were tested in vitro against two Gram-positive bacteria like Staphylococcus aureus, Bacillus subtilis; two Gram-negative bacteria like Escherichia coli, Pseudomonas aeruginosaand one fungal strain Candida albicans in comparison with standard drugs. Microbiological results showed that the synthesized compounds possessed a broad spectrum of antibacterial and antifungal activity against the tested microorganisms. The compounds with a 6-chloro (SK5b), 7-chloro-6-fluoro(SK5d) and 6-nitro (SK5e) on 2-amino benzothiazole ring possessing azo linkage showed better antimicrobial activity; almost similar or less to that of standard drugs thus they could be promising candidates for novel drugs. The novel heterocyclic derivatives were characterized by Physical characterization (Melting point, TLC) and different Spectroscopy techniques (IR, 1H NMR and Mass spectroscopy).
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1331
Introduction
The major drawback of current treatment of infectious diseases are challenging due to resistance to antimicrobial agents and their side effects. Limited numbers of antimicrobials are available to treat infections caused by fungi and mycobacteria. Chemotherapy for cancer treatment, immunosuppressive drugs for treatment of autoimmune diseases and organ transplant recipients, and infections (such as AIDS) that alter the effectiveness of the host immune system render individuals at high risk for fungal infections and certain mycobacterial infections. Numbers of new infectious diseases have been discovered. So, there is an urgent need to develop novel drugs molecule, with fewer side effects, broad spectrum activity, better stability and drugs with shorter lengths for the treatment of infectious disease. Microbial infection indirectly invites another disease due to low immunity power during infectious conditions. In the global market there are many antimicrobial agents but they all have some or more drawbacks, so to remove these drawbacks it is necessary to develop new antimicrobial agents. Rationale behind this work is to develop a molecule having good anti-microbial activity.
Based on review of Literature Benzothiazole, Sulphonamide and Azo moieties show significant antibacterial and antifungal activity. Based on review of literature 2-amino Benzothiazole at 4, 5 & 6th position substitution to improve biological activities. Literature survey reveals that electron withdrawing or donating groups amend the liphophilicity of the test compounds, which in turn alters permeability across the bacterial cell membrane.1 From research article Bhusari K. et al., concluded that Benzothiazole moiety attached to sulphonamide to improve antibacterial and antifungal properties.2 From research article Chaudhari M. et al., reported that synthesize and screen the antibacterial activity of some new Benzothiazole derivatives containing azo group in their structure.3According to research article MKPENIE V. et al., reported that azo compound of naphthalene and use it to investigate its inhibitory effect on the biological activities of bacterial strains. The presence of azo group contributed more than 60% of the antibacterial activities exhibited by azo-2-naphthol on all the bacteria tested.4So, based on these important concepts to develop novel bulky molecules comprising Benzothiazole, Sulphonamide, Azo group as potent antibacterial and antifungal agents.
[adsense:468x15:2204050025]
MATERIAL AND METHODS
All the Chemicals and Solvents were obtained from E-Merck, India (AR grade) and were used further purification. The melting point of the synthesized compounds were determined in open capillary using VEEGO MELTING POINT APPARATUS model VMP-D and recorded in Celsius without correction. Purity of the compound was verified by precoated TLC plates (E- Merck Kieselgel 60 F254). The Infrared spectra for the synthesized compounds were recorded using SHIMADZU-FTIR 8400S spectrometer using KBr as a back ground. 1H NMR spectra of the synthesized compounds were taken using BRUKER Advance-II 400 NMR spectrometer using Tetramethyl silane as an internal standard. 1H NMR spectra were recorded with DMSO as a solvent & the chemical shift data were expressed as delta values related to TMS. Mass spectra of the synthesized compounds were taken using 2010EV LCMS SHIMADZU instrument at 70 eV.
EXPERIMENTAL PROCEDURE
|
|
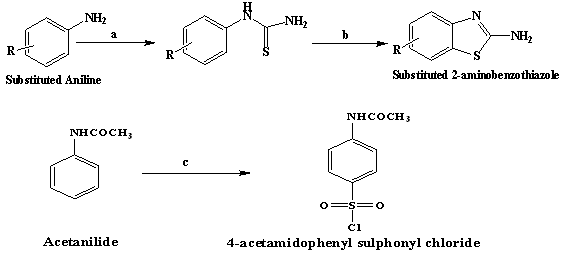
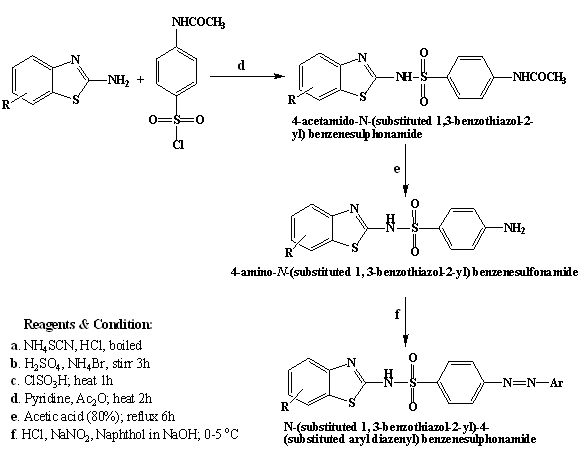
Figure 1: Route of synthesis
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Synthesis of Substituted 2-amino benzothiazole
To Substituted aniline (0.2742 mol), conc. hydrochloric acid (25 mL) was added and the solution was warmed. A saturated solution of ammonium thiocyanate in water (30g in 60 mL) was added slowly in above solution. The mixture was boiled until the solution got turbid. The turbid solution was poured in cold water. The separated precipitate as phenylthiourea was filtered and recrystallized from aqueous ethanol (80%) so as to obtain pure compound.1
Phenylthiourea (0.144 mol) was dissolved in sulphuric acid (45.39 mL, 85%) during half of an hour at 20 to 25 °C. In the period of 3 hours, 8.64 g of 40% NH4Br solution was then continuously metered in the resulting solution at 25 to 30 °C with stirring. The solution was then added to water, clarified by filtration and adjusted to pH 8 with sodium hydroxide solution. The mixture was stirred for half an hour at 40 °C and filtered using suction. The residue was washed free of sulfate with water, dried and recrystallized from aqueous ethanol.5
2-amino benzothiazole (SK1a)
Yield (%): 68.3; M.P. (OC): 128-134; Rf: 0.40 (Hexane : Ethyl acetate, 9:1).
6-chlorobenzothiazol-2-amine (SK1b)
Yield (%): 63.4; M.P. (OC): 194-196; Rf: 0.42 (Hexane : Ethyl acetate, 9:1).
6-methylbenzothiazol-2-amine (SK1c)
Yield (%): 61.8; M.P. (OC): 136-140; Rf: 0.41 (Hexane : Ethyl acetate, 9:1)
7-chloro-6-fluorobenzothiazol-2-amine (SK1d)
Yield (%): 72.5; M.P. (OC): 182-188; Rf: 0.36 (Hexane : Ethyl acetate, 9:1).
6-nitrobenzothiazol-2-amine (SK1e)
Yield (%): 73.6; M.P. (OC): 200-206; Rf: 0.39 (Hexane : Ethyl acetate, 9:1).
6-methoxybenzothiazol-2-amine (SK1f)
Yield (%): 65.6; M.P. (OC): 166-170; Rf: 0.41 (Hexane : Ethyl acetate, 9:1).
4,7-dimethoxybenzothiazol-2-amine (SK1g)
Yield (%): 59.2; M.P. (OC): 196-200; Rf: 0.47 (Hexane : Ethyl acetate, 9:1).
4,7-dichlorobenzothiazol-2-amine (SK1h)
Yield (%): 64.9; M.P. (OC): 164-168; Rf: 0.44 (Hexane : Ethyl acetate, 9:1).
7-chloro-4-methylbenzothiazol-2-amine (SK1i)
Yield (%): 62.6; M.P. (OC): 206-212; Rf: 0.46 (Hexane : Ethyl acetate, 9:1).
4-fluoro-7-nitrobenzothiazol-2-amine (SK1j)
Yield (%): 74.5; M.P. (OC): 214-218; Rf: 0.40 (Hexane : Ethyl acetate, 9:1).
Synthesis of 4-acetamidophenyl sulphonyl chloride (SK2)
To acetanilide (2.84 g, 0.021 mol) and good grade chlorosulphonic acid (7.3 mL) was added in small portions with constant stirring. The reaction mixture was heated on water bath for 1 hour then cooled and the oily mixture was poured in a thin stream with constant stirring into crushed ice (300 g). The lumps of the solid material were broken and the mixture was stirred for several minutes. The residue was filtered and washed with cold water.1
Yield (%): 67.8; M.P. (OC): 288-294; Rf: 0.50 (Hexane : Ethyl acetate, 9:1).
Synthesis of 4-acetamido-N-(substituted 1, 3-benzothiazol-2-yl) benzenesulphonamide
Substituted 2-amino benzothiazole (0.01 mol) was dissolved in stirring solution of pyridine (4 mL) and acetic anhydride (20 mL). To this mixture, 4-acetamidophenyl sulphonyl chloride (0.01 mol) was added. The mixture was heated on water bath for 2 hours. The reaction mixture was cooled and poured in cold water (20 mL). The solid obtained was filtered and crystallized from aqueous ethanol (80%) so as to obtain pure compound.1
4- acetamido-N- (benzothiazol-2-yl) benzenesulphonamide (SK3a)
Yield (%): 65.7; M.P. (OC): 188-190; Rf: 0.61 (Hexane : Ethyl acetate, 8:2).
4- acetamido-N-(6-chloro benzothiazol-2-yl) benzenesulphonamide (SK3b)
Yield (%): 63.2; M.P. (OC): 238-242; Rf: 0.60 (Hexane : Ethyl acetate, 8:2).
4- acetamido-N-(6-methyl benzothiazol-2-yl) benzenesulphonamide (SK3c)
Yield (%): 56.5; M.P. (OC): 194-198; Rf: 0.64 (Hexane : Ethyl acetate, 8:2).
4- acetamido-N-(7-chloro, 6-fluoro benzothiazol-2-yl) benzenesulphonamide (SK3d)
Yield (%): 68.6; M.P. (OC): 230-236; Rf: 0.65 (Hexane : Ethyl acetate, 8:2).
4- acetamido-N-(6-nitro benzothiazol-2-yl) benzenesulphonamide (SK3e)
Yield (%): 61; M.P. (OC): 242-248; Rf: 0.64 (Hexane : Ethyl acetate, 8:2).
4- acetamido-N-(6-methoxy benzothiazol-2-yl) benzenesulphonamide (SK3f)
Yield (%): 63.5; M.P. (OC): 206-210; Rf: 0.62 (Hexane : Ethyl acetate, 8:2).
4- acetamido-N-(4, 7-dimethoxy benzothiazol-2-yl) benzenesulphonamide (SK3g)
Yield (%): 63.5; M.P. (OC): 222-224; Rf: 0.66 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3326 (-NH-); 1722 (C=O), 1668 (C=N), 1349, 1125 (SO2), 1051 (C-O), 678 (C-S); 1H- NMR (DMSO, ppm): 12.35 (s, 1H, NHSO2), 11.24 (s, 1H, NHCO), 6.80- 7.90 (m, 6H, Ar-H), 2.57 (s, 3H, COCH3), 1.95 (s, 6H, OCH3); Mass spectra (m/z): 408.2 (M+1)+.
4- acetamido-N-(4, 7-dichloro benzothiazol-2-yl) benzenesulphonamide (SK3h)
Yield (%): 61.8; M.P. (OC): 212-216; Rf: 0.62 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3301 (NH); 1695 (C=O), 1646 (C=N), 1373, 1113 (SO2), 1279 (C-N), 711 (C-Cl), 668 (C-S).
4- acetamido-N-(4-methyl, 7-chloro benzothiazol-2-yl) benzenesulphonamide
Yield (%): 55.4; M.P. (OC): 234-238; Rf: 0.64 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3200 (NH), 1750 (C=O), 1640 (C=N), 1325, 1120 (SO2), 1300 (C-N), 750 (C-Cl), 680 (C-S).
4- acetamido-N-(4-fluoro, 7-nitro benzothiazol-2-yl) benzenesulphonamide
Yield (%): 70.6; M.P. (OC): 250-254; Rf: 0.67 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3357, 3259 (NH), 1699 (C=O), 1549 (C=N), 1506 (NO2), 1314, 1117 (SO2), 1076 (C-F); 642 (C-S); 1H- NMR (DMSO, ppm): 12.60 (s, 1H, NHSO2), 11.35 (s, 1H, NHCO), 7.07-8.57 (m, 6H, Ar-H), 2.52 (s, 3H, COCH3); Mass spectra (m/z): 412.3 (M+2)+.
Synthesis of 4-amino-N-(substituted1, 3-benzothiazol-2-yl) benzenesulphonamide
The compound 4-acetamido-N-(substituted1, 3-benzothiazol-2-yl) benzenesulphonamide(0.01 mol) was dissolved in acetic acid (80%, 50 mL). The solution was refluxed for 6 hours and cooled. The solution was poured in cold water (100 mL). The residue was filtered and washed with excess water and dried. The dried residue was recrystallized from aqueous ethanol (80%).1
4-amino-N-(benzothiazol-2-yl)benzenesulphonamide (SK4a)
Yield(%): 61.5; M.P. (°C): 166-170; Rf: 0.48 (Hexane : Ethyl acetate, 8:2).
4-amino-N-(6-chlorobenzothiazol-2-yl)benzenesulphonamide (SK4b)
Yield(%): 60.4; M.P. (°C): 212-218; Rf: 0.53 (Hexane : Ethyl acetate, 8:2).
4-amino-N-(6-methylbenzothiazol-2-yl)benzenesulphonamide (SK4c)
Yield(%): 55.3; M.P. (°C): 162-164; Rf: 0.52 (Hexane : Ethyl acetate, 8:2).
4-amino-N-(7-chloro-6-fluorobenzothiazol-2-yl)benzenesulphonamide (SK4d)
Yield(%): 62.7; M.P. (°C): 204-210; Rf: 0.54 (Hexane : Ethyl acetate, 8:2).
4-amino-N-(6-nitrobenzothiazol-2-yl)benzenesulphonamide (SK4e)
Yield(%): 61.8; M.P. (°C): 220-224; Rf: 0.57 (Hexane : Ethyl acetate, 8:2).
4-amino-N-(6-methoxybenzothiazol-2-yl)benzenesulphonamide (SK4f)
Yield(%): 62.6; M.P. (°C): 180-184; Rf: 0.55 (Hexane : Ethyl acetate, 8:2).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Synthesis of N-(substituted 1, 3-benzothiazol-2-yl)-4-(substituted aryl diazenyl) benzenesulphonamide
0.01 mol of 4-amino-N-(substituted1, 3-benzothiazol-2-yl) benzenesulphonamide was added in 20 ml Hydrochloric acid (50%), stirred and cooled to 0 °C to 5 °C. 8 mL of aqueous sodium nitrite (10.88 mmole, 0.7507 g) was added in drops maintaining the temperature between 0-5 °C. The diazonium chloride formed was consecutively coupled with 0.01 mol of 1-Naphthol or 2-Naphthol or Phenol that was dissolved in 15 mL of 10% sodium hydroxide solution. The reaction mixture was stirred at 0 °C to 5 °C for 1 hour. The product that precipitated was recrystallized from ethanol (65%).6
N-(benzothiazol-2-yl)-4-((4-hydroxynaphthalen-1-yl) diazenyl) benzenesulphonamide (SK5a)
Yield (%): 64.8; M.P. (°C): 262-264; Rf: 0.72 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3600 (OH), 3250 (NH), 1630 (C=N), 1430 (N=N), 1370, 1140 (SO2), 1300 (C-N), 650 (C-S); 1H- NMR (DMSO, ppm): 12.60 (s, 1H, NHSO2), 6.59- 8.21 (m, 14H, Ar-H), 5.60 (s, 1H, Ar-OH); Mass spectra (m/z): 462 (M+1)+.
N-(6-chlorobenzothiazol-2-yl)-4-((2-hydroxynaphthalen-1-yl) diazenyl) benzenesulphonamide (SK5b)
Yield (%): 63.8; M.P. (°C): 270-274; Rf: 0.75 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3300 (OH), 3200 (NH), 1652 (C=N), 1400 (N=N), 1375, 1090 (SO2), 1292(C-N), 707 (C-Cl), 608 (C-S); 1H- NMR (DMSO, ppm): 11.90 (s, 1H, NHSO2), 9.97 (s, 1H, Ar-OH), 7.22-8.30 (m, 13H, Ar-H); Mass spectra (m/z): 497.2 (M+2)+.
4-((2-hydroxynaphthalen-1-yl) diazenyl)-N-(6-methylbenzthiazol-2-yl) benzenesulphonamide (SK5c)
Yield (%): 59.7; M.P. (°C): 254-258; Rf: 0.68 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1):
3675 (OH), 3200 (NH), 1635 (C=N), 1410 (N=N), 1360, 1100 (SO2), 1275(C-N), 711 (C-S); 1H- NMR (DMSO, ppm): 12.18 (s, 1H, NHSO2), 8.81 (s, 1H, Ar-OH), 7.40-8.28 (m, 13H, Ar-H), 2.20 (s, 3H, CH3); Mass spectra (m/z): 475.4 (M)+.
N-(7-chloro-6-fluorobenzohiazol-2-yl)-4-((2-hydroxynaphthalen-1-yl) diazenyl) benzenesulphonamide (SK5d)
Yield (%): 73.1; M.P. (°C): 274-280; Rf: 0.76 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1):
3310 (OH), 3150 (NH), 1652 (C=N), 1447 (N=N), 1368, 1197 (SO2), 1268(C-N), 1033 (C-F), 715 (C-Cl), 667 (C-S); 1H- NMR (DMSO, ppm): 12.30 (s, 1H, NHSO2), 9.81 (s, 1H, Ar-OH), 6.97- 8.30 (m, 12H, Ar-H); Mass spectra (m/z): 512.9 (M)+.
4-((4-hydroxyphenyl) diazenyl)-N-(6- nitrobenzothiazol-2-yl) benzenesulphonamide (SK5e)
Yield (%): 66.3; M.P. (°C): 284-288; Rf: 0.78 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3270 (OH), 3150 (NH), 1652 (C=N), 1480, 1337 (NO2), 1430 (N=N), 1375, 1140 (SO2), 690 (C-S); 1H- NMR (DMSO, ppm): 12.68 (s, 1H, NHSO2), 6.77-8.94 (m, 11H, Ar-H), 5.57 (s, 1H, Ar-OH); Mass spectra (m/z): 456.1 (M+1)+.
4-((2-hydroxynaphthalen-1-yl) diazenyl)-N-(6-methoxybenzothiazol-2-yl) benzenesulphonamide (SK5f)
Yield (%): 65.8; M.P. (°C): 270-276; Rf: 0.74 (Hexane : Ethyl acetate, 8:2); IR (KBr, cm-1): 3518(OH), 3334 (NH), 1653 (C=N), 1456 (N=N), 1339, 1144 (SO2), 1224 (C-O), 668 (C-S); 1H- NMR (DMSO, ppm): 12.10 (s, 1H, NHSO2), 9.75 (s, 1H, Ar-OH), 6.61-8.29 (m, 13H, Ar-H), 2.06 (s, 3H, OCH3); Mass spectra (m/z): 491.7 (M)+.
BIOLOGICAL EVALUATION
The microbiological assay is based upon a comparison of inhibition of growth of microorganisms by measured concentrations of test compounds with that produced by known concentration of a standard antibiotic. Two methods generally employed are turbidometric (tube-dilution) method and cylinder plate (cup-plate) method. In the turbidometric method inhibition of growth of microbial culture in a uniform ablution of antibiotic in a fluid medium is measured. It is compared with the synthesized compounds. Here the presence or absence of growth is measured. The cylinder plate method depends upon diffusion of antibiotic from a vertical cylinder through a solidified agar layer in a Petridis or plate to an extent such that growth of added micro-organisms is prevented entirely in a zone around the cylinder containing solution of the antibiotics. The cup-plate method is simple and measurement of inhibition of microorganisms is also easy. Name of organisms for antimicrobial activity Gram Positive microorganisms [Staphylococcus aureus (MTCC No. 88), Bacillus subtilis (MTCC No. 10518)]; Gram Negative microorganisms [Escherichia coli (MTCC No. 44), Pseudomonas aeruginosa(MTCC No. 3541)]; Name of organisms for antifungal activity [Candida albicans (MTCC No. 10231)]
Preparation of test solution:
10 mg of the test compound was dissolved in 10 mL of DMSO. From this 2 mL of solution was taken and diluted to 10 mL with DMSO. Now the concentration of the test compound is 200 µg/mL. From this 5 mL of solution was taken and diluted to 10 mL with DMSO. Now the concentration of the test compound is 100 µg/mL. These sample solution were made in suitably labeled sterilized test tubes.
Preparation of standard solution:
The standard drug used for the testing is Ciprofloxacin. It is water soluble; the concentration of this drug is adjusted so as to contain 100 µg/mL and 200 µg/mL.The standard drug Griseofulvin was dissolve in appropriate quantity of Methanol to obtain the concentration of 100 μg/mL and 200 μg/mL.
Minimum inhibitory concentration test
This is the lowest concentration of the drug that will inhibit the growth and the replicating of the invading pathogen. This test is performed as a Tube Dilution Test. A standard amount of bacteria is placed within a series of tubes that have been diluted with a specific antibiotic or antimicrobial. These tubes are incubated overnight at 37 °C. No cloudiness means; no bacterial growth, whereas if the broths are cloudy, there is the presence of bacterial growth. Moving from right to left, you check the smaller dilutions of the broth for cloudiness. The first tube after the last cloudy tube is the MIC value.7
RESULTS AND DISCUSSION
All synthesized compounds were evaluated for antibacterial activity against E. coli, P. aeruginosa, B. subtilis, S. aureus andantifungal activity against C. albicans.
Table 1. Zone of Inhibition of Synthesized compounds for antibacterial and antifungal activity
|
Comp. Code
|
Diameter of zone of inhibition (mm) |
|||||||||
|
S. aureus |
B. subtitis |
E. coli |
P. aeruginosa |
C. albicans |
||||||
|
Conc. (µg/mL) |
100 |
200 |
100 |
200 |
100 |
200 |
100 |
200 |
100 |
200 |
|
SK3g |
8 |
14 |
- |
9 |
7 |
11 |
- |
9 |
- |
- |
|
SK3h |
9 |
13 |
- |
10 |
7 |
14 |
- |
9 |
- |
8 |
|
SK3i |
- |
8 |
- |
9 |
- |
8 |
- |
10 |
- |
- |
|
SK3j |
9 |
12 |
- |
10 |
9 |
13 |
- |
8 |
- |
12 |
|
SK5a |
10 |
15 |
9 |
14 |
11 |
15 |
10 |
14 |
- |
11 |
|
SK5b |
15 |
19 |
13 |
17 |
15 |
19 |
14 |
20 |
10 |
15 |
|
SK5c |
11 |
15 |
10 |
16 |
9 |
13 |
12 |
16 |
9 |
13 |
|
SK5d |
16 |
21 |
14 |
19 |
17 |
21 |
15 |
20 |
14 |
21 |
|
SK5e |
13 |
19 |
14 |
17 |
12 |
16 |
13 |
18 |
12 |
16 |
|
SK5f |
11 |
17 |
10 |
15 |
13 |
19 |
11 |
15 |
14 |
17 |
|
Ciprofloxacin |
23 |
28 |
21 |
29 |
25 |
33 |
24 |
30 |
- |
- |
|
Griseofulvin |
- |
- |
- |
- |
- |
- |
- |
- |
22 |
29 |
(-) indicates no inhibition zone (no activity)
Table 2. Minimum inhibitory concentration of synthesized compounds
|
Comp. Code |
Minimum inhibitory concentration (μg/mL) |
||||
|
S. aureus |
B. subtitis |
E. coli |
P. aeruginosa |
C. albicans |
|
|
SK3g |
62.5 |
125 |
62.5 |
125 |
250 |
|
SK3h |
62.5 |
125 |
62.5 |
125 |
125 |
|
SK3i |
125 |
125 |
125 |
125 |
250 |
|
SK3j |
62.5 |
125 |
62.5 |
125 |
125 |
|
SK5a |
62.5 |
62.5 |
62.5 |
62.5 |
125 |
|
SK5b |
31.25 |
31.25 |
31.25 |
31.25 |
62.5 |
|
SK5c |
62.5 |
62.5 |
62.5 |
62.5 |
62.5 |
|
SK5d |
31.25 |
31.25 |
15.5 |
31.25 |
31.25 |
|
SK5e |
31.25 |
62.5 |
31.25 |
31.25 |
62.5 |
|
SK5f |
62.5 |
62.5 |
31.25 |
62.5 |
62.5 |
|
Ciprofloxacin |
7 |
<5 |
<5 |
7 |
- |
|
Griseofulvin |
- |
- |
- |
- |
7 |

Figure 2: Biological activity (Zone of inhibition) of synthesized compounds
The objective of the present work was to Synthesis and biological evaluation of Bulky molecules comprising Benzothiazole and Sulphonamide moieties as antimicrobial agents. The yield of different synthesized compounds was found to be in the range of 59-72%. The physical characterization was done by melting point and TLC and spectral characterization was done by FTIR, 1H NMR, and Mass spectral studies.
From the in vitro antibacterial activity studies against the Gram-positive bacteria Staphylococcus aureus, Bacillus subtilus, the Gram-negative bacteria Escherichia coli, Pseudomonas aeruginosa and antifungal activity studies against Candida albicans,it was found that the synthesized compounds showed significant activity. Hence these synthesized compounds appear to be promising antibacterial and antifungal agents. The standard drug used was Ciprofloxacin for antibacterial and Griseofulvin as standard drug for antifungal activity.
Among the all synthesized compounds, compound 5dwith MIC value of (31.25 μg/mL) for S. aureus, (31.25 μg/mL) for B. subtilis, (15.5 μg/mL) for E. coli and (31.25 μg/mL) forP. aeruginosa gives better antibacterial activity than other synthesized compounds. And other compounds SK5b and SK5e with MIC value of (31.25 μg/mL) for S. aureus, (31.25 μg/mL, 62.5 μg/mL) for B. subtilis, (31.25 μg/mL) for E. coli and (31.25 μg/mL) forP. aeruginosa give good antibacerial activity. The compoundSK5d with MIC value of (31.25 μg/mL) against fungi (C. albicans) gives a better antifungal activitythan other synthesized compounds.
The in vitro antibacterial and antifungal data in the(Table 1, 2) indicate that the all the synthesized compounds were found to posses significant antibacterial and antifungal activity. Perhaps the presence of strong electron withdrawing groups such as -F, -Cl, and -NO2 at 6th position on 2-amino benzothiazole (SK5b, SK5d, and SK5e)showed highly significant antibacterial and antifungal activity. The presence of electron donating group such as -CH3 at6th position on 2-amino benzothiazole (SK5c) shown moderate activity. Based on in vitro antibacterial activity and antifungal activity, synthesized compounds SK5a to SK5f(Azo derivatives) showed better antibacterial and antifungal activity than SK3g to SK3j (Acetamide derivatives).Overall none of the compounds showed higher activity than the standards.
CONCLUSION
Result of present study demonstrate that, a new class of different Bulky molecules comprising Substituted Benzothiazole and Sulphonamide were synthesized and evaluated for antimicrobial activity by agar disk diffusion method. All the newly synthesized compounds showed moderate to good potency against different bacterial strains and fungal strain. Among them SK5b, SK5d and SK5e showed better antimicrobial activity. It can be concluded that this class of compounds certainly holds great promise towards good active leads in medicinal chemistry. A further study to acquire information concerning biological activity is in progress.
REFERENCES
1. Bhusari K, Amnerkar N, Khedekar P, Kale M “Synthesis and in vitro Antimicrobial Activity of Some New 4-Amino-N-(1, 3-Benzothiazol-2-yl) benzenesulphonamide Derivatives.” Asian J. Res. Chem. 2008, 1(2), 53-59.
2. Bhusari S, Pawar R and Vibute Y, Synthesis and Anti-microbial activity of 2-(substituted phenylsulfonamido)-6-substituted benzothiazoles.” Indian J. Hetero. Chem., 2001, 11, 79-88.
3. Chaudhary M, Pareek D, Ojha K and Pareek A, “Synthesis and Antibacterial Activity of 1-(2-Diazo-6-ethoxybenzothiazolyl) Substituted Benzene Derivatives.” Int. J. Curr. Chem., 2010, 1(3), 175-179.
4. Mkpenie V, Ebong G, Obot I and Abasiekong B, “Evaluation of the Effect of Azo Group on the Biological Activity of 1-(4-Methylphenylazo)-2-naphthol.” E-Journal of Chemistry, 2008, 5(3), 431-434.
5. Soni B, Bhandari A, Ranawat M, Sharma P, Sharma S and Prajapat R, “Synthesis and antimicrobial activity of some 2-substituted benzothiazoles containing azomethine linkage.” Pharmacophore, 2011, 2 (1), 36-45.
6. Eaton DC., Laboratory Investigation in Organic Chemistry, McGraw-Hill Inc, USA, 1989, pp 429-435.
7. Pelczar MJ., Chan ECS., and Krieg NR. In Microbiology; 5th Edn; Tata McGraw Hill Publishing Company Limited, New Delhi, 1993, pp 73-98.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE