About Authors:
Rajak Sandeep1, Kothiyal Preeti2, Mathur Prashant1
1Department of clinical pharmacy,
2Department of pharmacology,
Division of Pharmaceutical sciences,
Shri Guru Ram Rai Institute of Technology And Sciences, Patel Nagar, Dehradun-248001, Uttarakhand, India
sandeep.rajak1886@gmail.com
Abstract
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are widely prescribed for the treatment of hypertension and heart failure, as well as for kidney disease prevention in patients with diabetes mellitus and the management of patients after myocardial infarction.Both combination therapies of an Angiotensin II receptor blocker (ARB) with the thiazide hydrochlorothiazide (HCTZ) and an Angiotensin converting enzymes (ACEs) with HCTZ are recommended to achieve blood pressure (BP) goals in antihypertensive treatment. However, although HCTZ is known to have unfavorable effects on lipid metabolism, the effects of HCTZ in the ARB+HCTZ and ACEs+HCTZ combination on lipid metabolism have not been fully elucidated. The objective of present study was To study the Efficacy and Effects on Lipid Metabolism, anthropometry and blood pressure of Combination Treatment With ARB+HCTZ and ACEs+HCTZ in hypertensive patient.
All prescription of hypertensive patients coming to the medicine OPD and IPD would be screened and those prescribed with ACE-inhibitors+HCTZ and ARBs+HCTZ combination of drugs will be selected for this study.
REFERENCE ID: PHARMATUTOR-ART-2084
Inclusion criteria
- Patient at the age above 18 years or below 80 years.
- Patient suffering from BP and who are prescribed with ACE-inhibitor+HCTZ and ARBs+HCTZ combination of drugs.
Exclusion criteria
- Patient below 18 years or above 80 years.
- Patient co-prescribed with other antihypertensive drug.
On follow up a significant changes observed in systolic and diastolic blood pressure (p=0.0001) where as no significant changes was observed in lipid level as well as BMI or waist to hip ratio. Thus it concluded that ARBs+HCTZ was more efficacious in decreasing SBP than was ACEs+HCTZ combination in the management of hypertension. The unfavorable effect on lipid metabolism and BMI were observed with either combination.
INTRODUCTION
Hypertension
Hypertension (HTN) is the term used to denote elevated blood pressure (BP). It is defined as the condition in which BP remains consistent to systolic blood pressure (SBP) >140 mmHg and diastolic blood pressure (DBP) >90 mmHg. HTN can lead to heart disease, kidney disease or blindness and stroke. High BP is called “The Silent Killer” because it usually has no symptoms. It is one of the most common cardiovascular diseases in America and Worldwide [1].
Systolic Blood Pressure. The systolic pressure (the first and higher number) is the force that blood exerts on the artery walls as the heart contracts to pump out the blood. High systolic pressure is now known to be a greater risk factor than diastolic pressure for heart, kidney, and circulatory complications and for death, particularly in middle−aged and elderly adults. The wider the spread between the systolic and diastolic measurements, the greater the danger [2].
In fact, elevated systolic pressure may pose a significant danger for heart events and stroke events even when diastolic is normal −− a condition called isolated systolic hypertension. Isolated systolic hypertension is the most common form of hypertension in people older than fifty. In one study it comprised 87% of hypertension cases in people between ages 50 and 59.
Diastolic Blood Pressure. The diastolic pressure (the lower and second number) is the measurement of force as the heart relaxes to allow the blood to flow into the heart. High diastolic pressure (the second and lower number) is a strong predictor of heart attack and stroke in young adults[2].
Pulse Pressure. Pulse pressure is the difference between the systolic and the diastolic readings. It appears to be an indicator of stiffness and inflammation in the blood−vessel walls. The greater the difference between systolic and diastolic numbers, the stiffer and more injured the vessels are thought to be. Although not yet used by physicians to determine treatment, evidence is suggesting that it may prove to be a strong predictor of heart problems, particularly in older adults. Some studies suggest that in people over 45 years old, every 10−mm Hg increase in pulse pressure increases the risk for stroke increases by 11%, cardiovascular disease by 10%, and overall mortality by 16%. (In younger adults the risks are even higher.)[3].
- The heart pumps blood with excessive force.
- The body's smaller blood vessels (known as the arterioles) narrow, so that blood flow exerts more pressure against the vessels' walls.
Although the body can tolerate increased blood pressure for months and even years, eventually the heart may enlarge (a condition called hypertrophy), which is a major factor in heart failure. Such pressure can also injure blood vessels in the heart, kidneys, the brain, and the eyes.
Blood pressure is measured in millimeters of mercury (mm Hg). For example, excellent blood pressure would be less than 120/80 mm Hg (systolic/diastolic). Blood pressure is now categorized as optimal, normal, high normal, and hypertensive. The hypertensive category is further divided, according to severity [2].
Drug classification
Angiotensin converting enzyme. [ACE-Inhibitor]
Angiotensin converting enzyme (ACE-inhibitors) inhibitors block an enzyme that narrows blood vessels. This makes blood vessels relax and widen, reducing blood pressure. ACE -inhibitors inhibitAngiotensin-converting enzyme (a component of the blood pressure-regulating renin-angiotensin system), thereby decreasing the tension of blood vessels and blood volume, thus lowering blood pressure. ACE inhibitors are used primarily to treat hypertension, although they may also be prescribed for cardiac failure, diabetic nephropathy, chronic renal failure, renal involvement in systemic sclerosis.
The ability to reduce levels of angiotensin II with orally effective inhibitors of angiotensin converting enzyme (ACE) represents an important advance in the treatment of hypertension. Captopril (CAPOTEN) was the first such agent to be developed for the treatment of hypertension. Since then, enalapril (VASOTEC), lisinopril (PRINIVIL), quinapril (ACCUPRIL), ramipril (ALTACE), benazepril (LOTENSIN), moexipril (UNIVASC), fosinopril (MONOPRIL), trandolapril (MAVIK), and perindopril (ACEON) also have become available. These drugs have proven to be very useful for the treatment of hypertension because of their efficacy and their very favorable profile of adverse effects, which enhances patient adherence [4].
The ACE inhibitors appear to confer a special advantage in the treatment of patients with diabetes, slowing the development and progression of diabetic glomerulopathy. They also are effective in slowing the progression of other forms of chronic renal disease, such as glomerulosclerosis, and many of these patients also have hypertension. An ACE inhibitor is the preferred initial agent in these patients [4].
The endocrine consequences of inhibiting the biosynthesis of angiotensin II are of importance in a number of facets of hypertension treatment. Because ACE inhibitors blunt the rise in aldosterone concentrations in response to Na+ loss, the normal role of aldosterone to oppose diuretic-induced natriuresis is diminished. Consequently, ACE inhibitors tend to enhance the efficacy of diuretic drugs. This means that even very small doses of diuretics may substantially improve the antihypertensive efficacy of ACE inhibitors; conversely, the use of high doses of diuretics together with ACE inhibitors may lead to excessive reduction in blood pressure and to Na+ loss in some patients [4].
Common adversedrugreactions include:
hypotension, cough, hyperkalemia, headache, dizziness, fatigue, nausea, and renal impairment.
Angiotensin II Receptor Blocker. [ARBs]
Angiotensin II is a very potent chemical that causes muscles surrounding blood vessels to contract, thereby narrowing blood vessels. This narrowing increases the pressure within the vessels and can cause high blood pressure (hypertension).Angiotensin II receptor blockers (ARBs) are medications that block the action of angiotensin II by preventing angiotensin II from binding to angiotensin II receptors on blood vessels.ARBs are used for controlling high blood pressure, treating heart failure, and preventing kidney failure in people with diabetes or high blood pressure.
There are two distinct subtypes of angiotensin II receptors, designated as type 1 (AT1) and type 2 (AT2). The AT1 angiotensin II-receptor subtype is located predominantly in vascular and myocardial tissue and also in brain, kidney, and adrenal glomerulosa cells, which secrete aldosterone . The AT2 subtype of angiotensin II receptor is found in the adrenal medulla, kidney, and in the CNS, and may play a role in vascular development. Because the AT1 receptor mediates feedback inhibition of renin release, renin and angiotensin II concentrations are increased during AT1-receptor antagonism. The clinical consequences of increased angiotensin II effects on an uninhibited AT2 receptor are unknown; however, emerging data suggest that the AT2 receptor may elicit antigrowth and antiproliferative responses.
Therapeutic Uses:
When given in adequate doses, the AT1 receptor antagonists appear to be as effective as ACE inhibitors in the treatment of hypertension. As with ACE inhibitors, these drugs may be less effective in African-American and low-renin patients.
The full effect of AT1 receptor antagonists on blood pressure typically is not observed until about 4 weeks after the initiation of therapy. If blood pressure is not controlled by an AT1 receptor antagonist alone, a low dose of a hydrochlorothiazide or other diuretic may be added. In several randomized, double-blind studies of patients with mild-to-severe hypertension, the addition of hydrochlorothiazide to an AT1 receptor antagonist produced significant additional reductions in blood pressure in patients who demonstrated an insufficient response to hydrochlorothiazide alone. A smaller initial dosage is preferred for patients who have already received diuretics and therefore have an intravascular volume depletion, and for other patients whose blood pressure is highly dependent on angiotensin II. Given the different mechanisms by which they act, there is no assurance that the effects of ACE inhibitors and antagonists of the AT1 receptor will be equivalent in preventing target organ damage in patients with hypertension.
The most common side effects are cough, elevated potassium levels in the blood (hyperkalemia), low blood pressure, dizziness, headache, drowsiness, diarrhea, abnormal taste sensation (metallic or salty taste), and rash.Compared to ACE inhibitors, cough occurs less often with ARBs.
Thiazide hydrochlorothizide.[HCTZ]
Thiazide diuretics were the first tolerated efficient antihypertensive drugs that significantly reduced cardiovascular morbidity and mortality in placebo-controlled clinical studies. Although these drugs today still are considered a fundamental therapeutic tool for the treatment of hypertensive patient
Hydrochlorothiazide belongs to thiazide class of diuretics. It reduces blood volume by acting on the kidneys to reduce sodium (Na) reabsorption in the distal convoluted tubule. The major site of action in the nephron appears on an electroneutral Na+-Cl- co-transporter by competing for the chloride site on the transporter. By impairing Na transport in the distal convoluted tubule, hydrochlorothiazide induces a natriuresis and concomitant water loss. Thiazides increase the reabsorption of calcium in this segment in a manner unrelated to sodium transport [5]. Additionally, by other mechanisms, HCTZ is believed to lower peripheral vascular resistance[6].
Common Side Effects.Common side effects of diuretics are fatigue, depression, irritability, urinary incontinence, loss of sexual drive, breast swelling in men, and allergic reactions. Diuretics can trigger attacks of gout. They may also increase the risk of gastrointestinal (GI) bleeding. Diuretics may raise cholesterol level and, used alone, they have no effect on enlarged heart size (hypertrophy). Arrhythmias can also occur as an interaction between diuretics and certain drugs, including some antidepressants, anti−arrhythmic drugs themselves, and digitalis [5][6].
Objective
To study the Efficacy and Effects on Lipid Metabolism, anthropometry and blood pressure of Combination Treatment With ARB+HCTZ and ACEs+HCTZ.
Methodology
Study Design
This prospective study was conducted at a tertiary care hospital of Dehradun district in Uttarakhand. All prescription of hypertensive patients coming to the medicine OPD and IPD were screened and those prescribed with ACE inhibitors+HCTZ and ARBs+HCTZ combination of drugs were enrolled selected for this study.
Inclusion criteria
* Patient at the age above 18 years or below 80 years.
* Patient suffering from BP and who were prescribed with ACE-inhibitor+HCTZ and ARBs+HCTZ combination of drugs.
Exclusion criteria
* Patient below 18 years or above 80 years.
* Patient co-prescribed with other antihypertensive drug.
The patient were categorized into 2 groups on the basis of drug prescribed by qualified physician. Group A- ACE-inhibitor+HCTZ and groupB- ARBs+HCTZ. Following parameters were evaluate at the baseline (130/90. mm/hg) at (before start of the therapy).
* Antropromatary parameter
* Blood pressure (systolic and diastolic)
* Lipid parameter:
- Total lipid
- Phospholipids
- Triglyceride
- Total cholesterol
- HDL
- LDL
- VLDL
All the hypertensive and bio-chemical parameter of the patient were followed for 12 weeks to assess response to the therapy. The following parameter were re-assessed after 12 weeks of follow up.
- Weight
- Waist
- Hip
- Waist/hip ratio
- Blood pressure
- Lipid profile
Methods
* Anthropometric analysis
BMI= WEIGHT(kg)/HEIGHT2(in meters)
* Blood pressure
Blood pressure in mm/hg was recorded in sitting position with a mercury sphygmomanometer. Average of three reading five minute apart was taken as the final value.
* Lipid parameter
Total lipid profile in mg/dl including total cholesterol, TG,TC, LDL, HDL VLDL, TG/HDL, TC/HDL, LDL/HDL was evaluating by using biochemical analyzer.
RESULTS
The study was conducted on one hundred hypertensive patient. Out of which 95 patient completed 12 weeks of follow up. A total of 54 subjects randomly received treatments with ARBs+HCTZ out of 95 patients while 41 subjects received ACEs+HCTZ. The patients were diagnosed and prescribed medicines by a qualified physician. The data reported in this study is from the observation recorded in the individual prescription of individual patient, who met the inclusion criteria for being enrolled in the present study. Five patients excluded from this study due to lack of sufficient data.
Table 5.1- Distribution of male and female patient on the basis of age and sex in ARBs+HCTZ combination group.
|
Age distribution |
Male |
Female |
|
30-40 |
00 |
03 |
|
40-50 |
00 |
18 |
|
50-60 |
16 |
08 |
|
60-70 |
07 |
00 |
|
More then 70 |
04 |
00 |
Table Distribution of male and female patient on the basis of age and sex in ACE+HCTZ group.
|
Age distribution |
Male |
Female |
|
30-40 |
00 |
05 |
|
40-50 |
00 |
13 |
|
50-60 |
08 |
09 |
|
60-70 |
04 |
00 |
|
More then 70 |
02 |
00 |
* Baseline general characteristics of ARBs+HCTZ:
All the patients of ARBs+HCTZ were non obese (BMI?30) with mean BMI at the baseline 27.544±4.544 kg/mm2. The mean baseline of waist/hip ratio 0.9745±0.0948 and blood pressure mean systolic was 148.574±19.509 mm/hg and diastolic was 87.537±11.276 mm/hg. The other general characteristics are shown in table.
TABLE MEAN and SD of general characteristics at the baseline in all ARBs+HCTZ Patients.
|
GENERAL PARAMETER |
MEAN |
SD |
|
WT |
70.351 |
12.631 |
|
HT |
159.721 |
6.689 |
|
BMI |
27.544 |
4.544 |
|
WAIST |
40.981 |
10.445 |
|
HIP |
42.305 |
11.715 |
|
WAIST/HIP RATIO |
0.9745 |
0.0948 |
|
SYSTOLIC |
148.574 |
19.509 |
|
DIASTOLIC |
87.537 |
11.276 |
* Baseline characteristics of bio-chemical parameter of ARBs+HCTZ:
All the bio-chemical parameters are observed in patient before start of the therapy in this group were LDL (111.407±45.288), HDL (39.370±7.678), TG (158.259±61.671),TC (184.637±39.212), VLDL (32.844±17.1787), TG/HDL (4.314±2.207), TC/HDL (4.906±1.616), LDL/HDL (3.023±1.568).
TABLE MEAN and SD of biochemical parameter of ARBs+HCTZ patients at base line.
|
BIOCHEMICAL PARAMETER |
MEAN |
SD |
|
LDL |
111.407 |
45.288 |
|
HDL |
39.370 |
7.678 |
|
TG |
158.259 |
61.671 |
|
TC |
184.637 |
39.212 |
|
VLDL |
32.844 |
17.1787 |
|
TG/TDL |
4.314 |
2.207 |
|
TC/HDL |
4.906 |
1.616 |
|
LDL/HDL |
3.023 |
1.568 |
* Baseline general characteristics of ACE+HCTZ:
All the the patients of ACE+HCTZ were non obese (BMI?30) with mean BMI at the baseline 27.222±5.500 kg/mm2. The mean baseline of waist/hip ratio 0.9635±0.0771 and blood pressure mean systolic blood pressure was 147.414±15.728 mm/hg and diastolic blood pressure was 89.506±9.211 mm/hg. The other general characteristics at the baseline in ACEs+HCTZ shown in table.
TABLE MEAN and SD of general characteristics at the baseline in all ACE+HCTZ Patients.
|
GENERAL PARAMETER |
MEAN |
SD |
|
WT |
69.536 |
12.560 |
|
HT |
160.453 |
10.312 |
|
BMI |
27.222 |
5.500 |
|
WAIST |
39.951 |
9.238 |
|
HIP |
41.524 |
8.997 |
|
WAIST/HIP RATIO |
0.9635 |
0.0771 |
|
SYSTOLIC |
147.414 |
15.728 |
|
DIASTOLIC |
89.506 |
9.211 |
* Baseline characteristics of bio-chemical parameter of ACE+HCTZ:
All the bio-chemical parameters are observed in patient before start of the therapy in this group were LDL (117.487±46.044), HDL (37.174±8.232), TG (170.682±71.333),TC (190.0±39.756), VLDL (33.097±17.423), TG/HDL (4.955±2.554), TC/HDL (5.470±2.014), LDL/HDL (3.444±1.864).
TABLE MEAN and SD of biochemical parameter of ACE+HCTZ patients at base line.
|
BIOCHEMICAL PARAMETER |
MEAN |
SD |
|
LDL |
117.487 |
46.044 |
|
HDL |
37.174 |
8.232 |
|
TG |
170.682 |
71.333 |
|
TC |
190.0 |
39.756 |
|
VLDL |
33.097 |
17.423 |
|
TG/TDL |
4.955 |
2.554 |
|
TC/HDL |
5.470 |
2.014 |
|
LDL/HDL |
3.444 |
1.864 |
* Response of ABRs+HCTZ after 12 weeks of treatment in all patient of this group:
Table Response of ARBs+HCTZ after treatment:
|
GENERAL AND BIOCHEMICAL PARAMETER |
BASELINE VALUE |
FINAL VALUE |
T |
P-VALUE |
|
WT |
70.351± 12.631 |
69.407±12.036 |
|
|
|
HT |
159.721±6.689 |
159.721±6.689 |
|
|
|
BMI |
27.544±4.544 |
27.190±4.390 |
0.3727 |
0.5508 |
|
WAIST |
40.981±10.445 |
39.888±10.027 |
0.5829 |
0.5289 |
|
HIP |
42.305±11.715 |
41.351±11.2 |
0.4775 |
0.5187 |
|
WAIST/HIP RATIO |
0.9745±0.0948 |
0.9745±0.0948 |
0.3499 |
0.9786 |
|
SYSTOLIC |
148.574±19.509 |
135.907±6.355 |
4.927 |
< 0.0001 |
|
DIASTOLIC |
87.537±11.276 |
83.277±6.026 |
3.886 |
< 0.0001 |
|
LDL |
111.407±45.288 |
110.074±42.760 |
0.284 |
0.6877 |
|
HDL |
39.370±7.678 |
39.703±7.532 |
0.2224 |
0.8938 |
|
TG |
158.259±61.671 |
153.667±58.432 |
0.380 |
0.5507 |
|
TC |
184.637±39.212 |
180.32±37.836 |
0.5807 |
0.2947 |
|
VLDL |
32.844±17.1787 |
30.522±13.911 |
0.7684 |
0.2922 |
|
TG/HDL |
4.314±2.207 |
4.133±2.041 |
0.422 |
0.5207 |
|
TC/HDL |
4.906±1.616 |
4.740±1.540 |
0.5034 |
0.3637 |
|
LDL/HDL |
3.023±1.568 |
2.931±1.475 |
0.2952 |
0.6480 |
All the parameter were recorded after 12 weeks of treatement with the ARBs+HCTZ combination group. A changes in all the parameter were observed systolic pressure was 148.574±19.509??135.907±6.355 and diastolic pressure was 87.537±11.276??83.277±6.026. significant changes systolic and diastolic blood pressure were observed(Pvalue < 0.0001). while there is no significant changes were observed in general or biochemical parameter.
* Change in general and biochemical parameter after treatment with ARB+HCTZ.
All the average changes and % changes parameter observed after treatment were average change in BMI was 0.354755±1.273885, % changes was 1.123602±4.497008. The waist/hip ratio changes was (0.005493±0.029575) (0.378432±2.896548), the systolic and diastolic changes were (12.66667±15.32786) (7.482745±8.628532), (4.259259±7.258528)(4.046963±7.584676) respectively. The other parameters are show in the table.
Table Average changes and percentage changes after treatment in ARBs+HCTZ groups.
|
|
Average change |
% CHANGE |
|
BMI |
0.354755±1.273885 |
1.123602±4.497008 |
|
WAIST |
1.092593±1.032829 |
2.528678±2.267348 |
|
HIP |
0.953704±0.837046 |
2.129317±1.680983 |
|
WAIST/HIP RATIO |
0.005493±0.029575 |
0.378432±2.896548 |
|
SYSTOLIC |
12.66667±15.32786 |
7.482745±8.628532 |
|
DIASTOLIC |
4.259259±7.258528 |
4.046963±7.584676 |
|
LDL |
2.333333±3.256344 |
0.517292±8.244765 |
|
HDL |
-0.33333±1.098885 |
-1.01666±3.109783 |
|
TG |
4.592593±5.615069 |
2.391099±2.858435 |
|
TC |
4.337037±4.23816 |
2.241255±2.457693 |
|
VLDL |
2.322222±3.998946 |
4.635554±6.646536 |
|
TG/HDL |
0.181214±0.279968 |
3.263991±4.536748 |
|
TC/HDL |
0.165675±0.179917 |
3.153814±3.372052 |
|
LDL/HDL |
0.092465±0.15122 |
1.491528±7.47443 |
* Response of ACE+HCTZ after 90days of treatment in all patient of this group:
Table Response of ACEs+HCTZ after treatment:
|
GENERAL AND BIOCHEMICAL PARAMETER |
BASELINE VALUE |
FINAL VALUE |
T |
P-VALUE |
|
WT |
69.536±12.560 |
68.195±12.418 |
|
|
|
HT |
160.453±10.312 |
160.453±10.312 |
|
|
|
BMI |
27.222±5.500 |
26.711±5.514 |
0.468 |
0.5508 |
|
WAIST |
39.951±9.238 |
39.243±8.831 |
0.3288 |
0.5289 |
|
HIP |
41.524±8.997 |
40.512±8.479 |
0.4416 |
0.5187 |
|
WAIST/HIP RATIO |
0.9635±.0771 |
0.9696±.07158 |
0.3404 |
0.9786 |
|
SYSTOLIC |
147.414±15.728 |
135.292±4.971 |
4.108 |
< 0.0001 |
|
DIASTOLIC |
89.506±9.211 |
85.024±4.921 |
2.454 |
< 0.0001 |
|
LDL |
117.487±46.044 |
114.682±43.337 |
0.2865 |
0.6877 |
|
HDL |
37.174±8.232 |
37.146±7.799 |
0.01645 |
0.8938 |
|
TG |
170.682±71.333 |
164.292±60.328 |
0.4613 |
0.5507 |
|
TC |
190.0±39.756 |
182.390±38.568 |
0.8879 |
0.2947 |
|
VLDL |
33.097±17.423 |
30.560±13.985 |
0.7314 |
0.2922 |
|
TG/HDL |
4.955±2.554 |
4.716±2.156 |
0.4858 |
0.5207 |
|
TC/HDL |
5.470±2.014 |
5.180±1.713 |
0.769 |
0.3637 |
|
LDL/HDL |
3.444±1.864 |
3.319±1.641 |
0.3492 |
0.6480 |
All the parameter were recorded after 12 weeks of treatement with the ACEs+HCTZ combination group. A changes in all the parameter were observed systolic pressure was 147.414±15.728??135.292±4.971 and diastolic pressure was 89.506±9.211??85.024±4.921. significant changes systolic and diastolic blood pressure were observed(Pvalue < 0.0001). while there is no significant changes were observed in general or biochemical parameter in this group.
* Change in general and biochemical parameter after treatment with ACE+HCTZ.
Table Average changes and percentage changes after treatment in ACEs+HCTZ group.
|
|
Average change |
% CHANGE |
|
BMI |
0.511071±0.615137 |
1.936626±2.037631 |
|
WAIST |
0.707317±0.843917 |
1.630036±1.922184 |
|
HIP |
1.012195±0.869539 |
2.297779±1.843019 |
|
WAIST/HIP RATIO |
-0.00613±0.021846 |
-0.70662±2.25 |
|
SYSTOLIC |
12.12195±12.21105 |
7.580992±6.565862 |
|
DIASTOLIC |
4.536585±5.532168 |
4.592799±5.274931 |
|
LDL |
2.804878±3.815885 |
1.848055±2.056123 |
|
HDL |
0.028293±1.313324 |
-0.34518±4.507935 |
|
TG |
6.390244±13.66177 |
2.541287±4.018444 |
|
TC |
7.609756±15.38486 |
3.686417±7.181261 |
|
VLDL |
2.536585±3.886499 |
5.316558±7.141092 |
|
TG/HDL |
0.239432±0.508553 |
2.702878±5.701516 |
|
TC/HDL |
0.2904638±0.6839617 |
3.86718±7.960837 |
|
LDL/HDL |
0.125519±0.331439 |
2.005916±4.577881 |
All the average changes and % changes parameter observed after treatment were average change in BMI was 0.511071±0.615137,% changes was 1.936626±2.037631. The waist/hip ratio changes was (-0.00613±0.021846) (-0.70662±2.25), the systolic and diastolic changes were (12.12195±12.21105)(7.580992±6.565862),(4.536585±5.532168)(4.592799±5.274931) respectively. The other parameters are show in the table.
Comparison of study group:
Table Comparison of baseline parameter of ACEs+HCTZ and ARBs+HCTZ:
|
|
ARBs +HCTZ |
ACEs+HCTZ |
T value |
P value |
|
BMI |
27.544±4.544 |
27.222±5.500 |
t=0.3124 |
0.7554 |
|
WAIST |
40.981±10.445 |
39.951±9.238 |
t=0.03103 |
0.9753 |
|
HIP |
42.305±11.715 |
41.524±8.997 |
t=0.08079 |
0.9358 |
|
WAIST/HIP RATIO |
0.9745±0.0948 |
0.9635±.0771 |
t=0.3490 |
0.7279 |
|
SYSTOLIC |
148.574±19.509 |
147.414±15.728 |
t=4.883 |
< 0.0001 |
|
DIASTOLIC |
87.537±11.276 |
89.506±9.211 |
t=0.9360 |
0.3517 |
|
LDL |
111.407±45.288 |
117.487±46.044 |
t=0.5377 |
0.5921 |
|
HDL |
39.370±7.678 |
37.174±8.232 |
t=1.338 |
0.1841 |
|
TG |
158.259±61.671 |
170.682±71.333 |
t=0.9087 |
0.3658 |
|
TC |
184.637±39.212 |
190.0±39.756 |
t=0.6563 |
0.5132 |
|
VLDL |
32.844±17.1787 |
33.097±17.423 |
t=0.07070 |
0.9438 |
|
TG/HDL |
4.314±2.207 |
4.955±2.554 |
t=1.310 |
0.1933 |
|
TC/HDL |
4.906±1.616 |
5.470±2.014 |
t=1.514 |
0.1335 |
|
LDL/HDL |
3.023±1.568 |
3.444±1.864 |
t=1.193 |
0.2357 |
Table Comparison of follow up parameter of ACEs+HCTZ and ARBs+HCTZ:
|
|
ARBs +HCTZ |
ACEs+HCTZ |
T value |
P value |
|
BMI |
27.190±4.390 |
26.711±5.514 |
t=0.4712 |
0.6386 |
|
WAIST |
39.888±10.027 |
39.243±8.831 |
t=0.3267 |
0.7446 |
|
HIP |
41.351±11.2 |
40.512±8.479 |
t=0.4005 |
0.6897 |
|
WAIST/HIP RATIO |
0.9745±0.0948 |
0.9696±.07158 |
t=0.03486 |
0.9723 |
|
SYSTOLIC |
135.907±6.355 |
135.292±4.971 |
t=0.5116 |
0.6101 |
|
DIASTOLIC |
83.277±6.026 |
85.024±4.921 |
t=1.512 |
0.1340 |
|
LDL |
110.074±42.760 |
114.682±43.337 |
t=0.5173 |
0.6062 |
|
HDL |
39.703±7.532 |
37.146±7.799 |
t=1.614 |
0.1099 |
|
TG |
153.667±58.432 |
164.292±60.328 |
t=0.8657 |
0.3889 |
|
TC |
180.32±37.836 |
182.390±38.568 |
t=0.2645 |
0.7920 |
|
VLDL |
30.522±13.911 |
30.560±13.985 |
t=0.01342 |
0.9893 |
|
TG/HDL |
4.133±2.041 |
4.716±2.156 |
t=1.346 |
0.1817 |
|
TC/HDL |
4.740±1.540 |
5.180±1.713 |
t=1.311 |
0.1931 |
|
LDL/HDL |
2.931±1.475 |
3.319±1.641 |
t=1.208 |
0.2299 |
There were no significant changes observed between these ARB+HCTZ and ACEs+HCTZ groups.
Table Comparison of blood pressure in ARBs+HCTZ and ACE+HCTZ after 12 weeks of treatment in all patients of these groups:
|
ARBs+HCTZ |
ACE+HCTZ |
|||
|
|
Basal |
Follow up |
Basal |
Follow up |
|
Systolic |
148.574±19.509 |
135.907±6.355 |
147.414±15.728 |
135.292±4.971 |
|
Diastolic |
87.537±11.276 |
83.277±6.026 |
89.506±9.211 |
85.024±4.921 |
All the blood pressure parameter observed after the treatment in ARBs+HCTZ and ACE+HCTZ the basal and final values were systolic pressure 148.574±19.509, 135.907±6.355 andDiastolic pressure 87.537±11.276, 83.277±6.026. The ACE+HCTZ systolic pressure 147.414±15.728, 135.292±4.971. Diastolic pressure was 89.506±9.211, 85.024±4.921.
Figure-5.1: Systolic Blood Pressure:
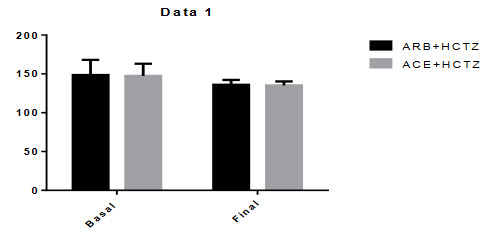
Mean differences of systolic blood pressure between basal to final of ARBs+HCTZ was 12.67 and where ACE+HCTZ was 12.12 . The p-value was P < 0.0001. There were significant changes in the reduction of systolic blood pressure from baseline during this combination therapy.
Figure-5.2: Diastolic Blood Pressure:
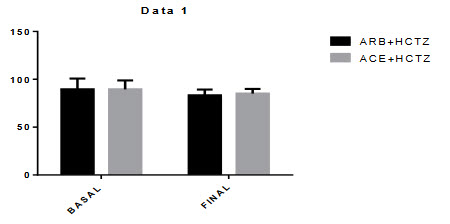
Mean differences of diastolic blood pressure between basal to final of ARBs+HCTZ was 6.259 and where ACE+HCTZ was 4.537 and the p-value was P < 0.0001.There were significant changes in the reduction of diastolic blood pressure from baseline during this combination therap
|
ARB+ HCTZ ACE+HCTZ |
||||
|
|
Basal |
Final |
Basal |
Final |
|
WT. |
70.351± 12.631 |
69.407±12.036 |
69.536±12.560 |
68.195±12.418 |
|
HT |
159.721±6.689 |
159.721±6.689 |
160.453±10.312 |
160.453±10.312 |
|
BMI |
27.544±4.544 |
27.190±4.390 |
27.222±5.500 |
26.711±5.514 |
|
WAIST |
40.981±10.445 |
39.888±10.027 |
39.951±9.238 |
39.243±8.831 |
|
HIP |
42.305±11.715 |
41.351±11.2 |
41.524±8.997 |
40.512±8.479 |
|
WAIST/HIP RATIO |
0.9745±0.0948 |
0.9691±0.0774 |
0.9635±0.0771 |
0.9696±0.07158 |
Table Comparison of general parameter in ARBs+HCTZ and ACE+HCTZ after 12 weeks of treatment in all patients of this group:
Figure 5.3: Figure Comparison of WAIST\HIP Ratio:
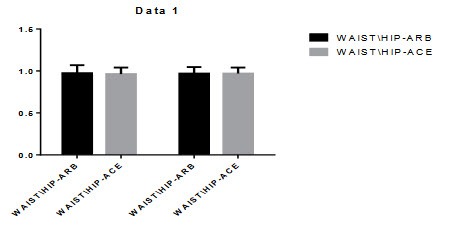
Figure 5.4: Comparison of BMI
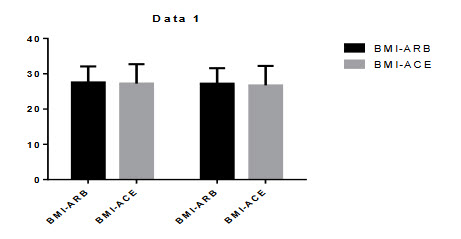
All the BMI and WAIST/HIP ratio observed after the treatment in ARBs+HCTZ and ACE+HCTZ the basal and final values were 27.544±4.544, 27.190±4.390. Andwaist/hip ratio was 0.9745±0.0948, 0.9691±0.0774. The ACE+HCTZ BMI was 27.222±5.500, 26.711±5.514. and waist/hip ratio was 0.9635±0.0771, 0.9696±0.07158. After the treatment there was no significant changes found in BMI and WAIST/HIP ratio.
Table 5.15:- Comparison of biochemical parameter in ARBs+HCTZ and ACE+HCTZ after 12 weeks of treatment in all patients of this group:
|
ARBs+HCTZ ACE+HCTZ |
||||
|
|
BASAL |
FINAL |
BASAL |
FINAL |
|
LDL |
111.407±45.288 |
110.074±42.760 |
117.487±46.044 |
114.682±43.337 |
|
HDL |
39.370±7.678 |
39.703±7.532 |
37.174±8.232 |
37.146±7.799 |
|
TG |
158.259±61.671 |
153.667±58.432 |
170.682±71.333 |
164.292±60.328 |
|
TC |
184.637±39.212 |
180.32±37.836 |
190.0±39.756 |
182.390±38.568 |
|
VLDL |
32.844±17.1787 |
30.522±13.911 |
33.097±17.423 |
30.560±13.985 |
|
TG/HDL |
4.314±2.207 |
4.133±2.041 |
4.955±2.554 |
4.716±2.156 |
|
TC/HDL |
4.906±1.616 |
4.740±1.540 |
5.470±2.014 |
5.180±1.713 |
|
LDL/HDL |
3.023±1.568 |
2.931±1.475 |
3.444±1.864 |
3.319±1.641 |
All the biochemical parameter observed after the treatment of ARBs+HCTZ combination group LDL (111.407±45.288)(110.074±42.760), HDL (39.370±7.678)(39.703±7.532), TG(158.259±61.671)(153.667±58.432), TC(184.637±39.212)(180.32±37.836), TG/HDL(4.314±2.207)(4.133±2.041), TC/HDL(4.906±1.616)(4.740±1.540), LDL/HDL(3.023±1.568)(2.931±1.475). ACEs+HCTZ wasLDL(117.487±46.044)(114.682±43.337), HDL(37.174±8.232)(37.146±7.799), TG(170.682±71.333)(164.292±60.328), TC(190.0±39.756)(182.390±38.568), TG/HDL(4.955±2.554)(4.716±2.156), TC/HDL(5.470±2.014)(5.180±1.713),LDL/HDL(3.444±1.864)(3.319±1.641).
In both the combination group resulted there is no significant differences in the reduction of the biochemical parameter.
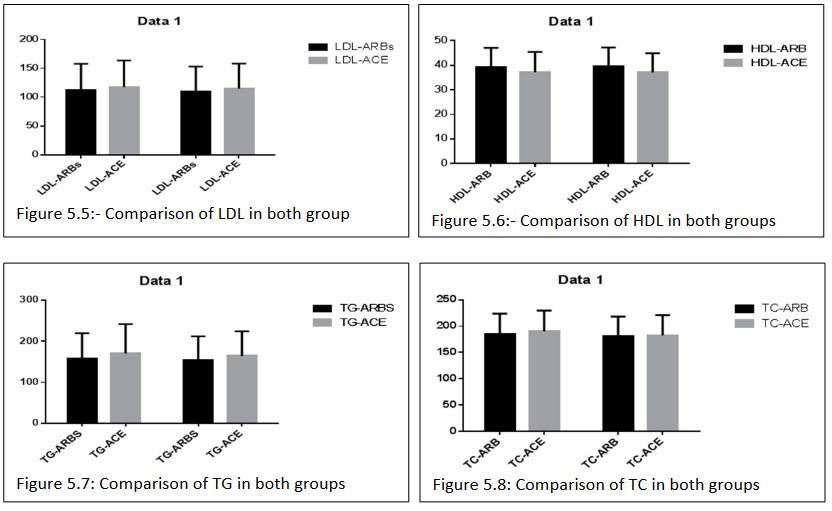
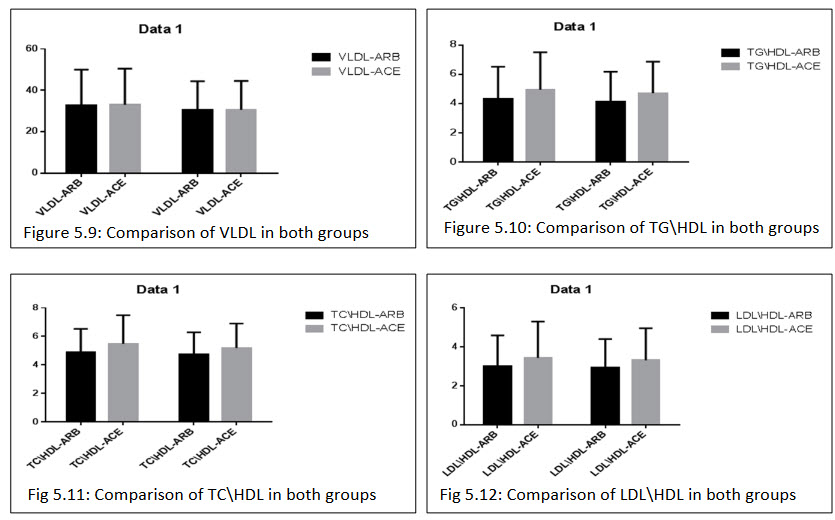
Table 5.16: Comparison of percentage changes in general and biochemical parameters after 12 weeks of treatment in both the groups:
|
|
ARBs +HCTZ |
ACEs+HCTZ |
T value |
P value |
|
BMI |
1.123602±4.497008 |
1.936626±2.037631 |
t=1.076 |
0.2848 |
|
WAIST |
2.528678±2.267348 |
1.630036±1.922184 |
t=2.041 |
0.0441 |
|
HIP |
2.129317±1.680983 |
2.297779±1.843019 |
t=0.4641 |
0.6437 |
|
WAIST/HIP RATIO |
0.378432±2.896548 |
-0.70662±2.25 |
t=1.986 |
0.0500 |
|
SYSTOLIC |
7.482745±8.628532 |
7.580992±6.565862 |
t=0.06074 |
0.9517 |
|
DIASTOLIC |
4.046963±7.584676 |
4.592799±5.274931 |
t=0.3939 |
0.6946 |
|
LDL |
0.517292±8.244765 |
1.848055±2.056123 |
t=1.009 |
0.3157 |
|
HDL |
-1.01666±3.109783 |
-0.34518±4.507935 |
t=0.8587 |
0.3927 |
|
TG |
2.391099±2.858435 |
2.541287±4.018444 |
t=0.2129 |
0.8319 |
|
TC |
2.241255±2.457693 |
3.686417±7.181261 |
t=1.378 |
0.1714 |
|
VLDL |
4.635554±6.646536 |
5.316558±7.141092 |
t=0.4790 |
0.6331 |
|
TG/HDL |
3.263991±4.536748 |
2.702878±5.701516 |
t=0.5342 |
0.5945 |
|
TC/HDL |
3.153814±3.372052 |
3.86718±7.960837 |
t=0.5929 |
0.5547 |
|
LDL/HDL |
1.491528±7.47443 |
2.005916±4.577881 |
t=0.3885 |
0.6985 |
There were no significant changes observed in percentage of general and biochemical parameter of these groups.
Discussion
Cardiovascular risk reduction with an ARB/ACE inhibitor with HCTZ have been studied previously. The benefits of ARBs+HCTZ and ACEs+ HCTZ were recently compared in the ACCOMPLISH (Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension) trial (Jamerson et al 2008). In the COPE (Combination Therapy of Hypertension to Prevent Cardiovascular Events) trial, (Matsuzaki et al 2011). Combination treatments with a ARB+HCTZ and ACEs+HCTZ were observed to have similar efficacy in the prevention of cardiovascular events and the achievement of target BP. Thus, in terms of efficacy in BP lowering and cardiovascular risk reduction, the benefits of the 2 groups therapies seem to still be debatable.
The present study compared the effects on lipid metabolism and blood pressure between Angiotensin receptor blocker+ HCTZ and ACEs+ HCTZ in patients who are suffering from the hypertension.
The results of this study demonstrated that the ARB+HCTZ combination was significantly more effective at reducing SBP than ACEs+HCTZ combination after 12 weeks of therapy in stage1and 2 hypertensive patients. Responder rates for SBP < 140 mmHg were significantly greater at Week 2 in the group, suggesting a more rapid onset of action, and were numerically greater in the group at Weeks 6 and 8, with p-values nearing statistical significance. Both treatments were generally well tolerated. These results support the recommendations of the (JNC-7 2010), the (European Society of Hypertension 2003), and the Taiwan Society of Cardiology Guidelines for the Management of Hypertension, which advocate early initiation of combination treatment consisting of two anti-hypertensive drugs with complimentary mechanisms of action for stage 2 hypertension patients (Neutel et al 2005),( Dahlof et al 2002).
A target level of <140/90 mmHg is recommended for patients with uncomplicated hypertension and no evidence of diabetes or renal disease (Chobanian et al 2003; European Society of Hypertension 2003; Whitworth 2003). Importantly, in an open-label trial, 9 out of 10 patients with Stage 1 hypertension, and more than half of patients with Stage 2 hypertension achieved an aggressive BP goal of ≤130/ 85 mmHg when treated with olmesartan medoxomil/HCTZ (Neutel et al 2006).
Mean differences of systolic blood pressure between basal to final of ARBs+HCTZ was 12.67 where ACE+HCTZ was 12.12 and the p-value was P < 0.0001. Mean differences of diastolic blood pressure between basal to final of ARBs+HCTZ was 6.259 and where ACE+HCTZ was 4.537 and the p-value was P < 0.0001. BP was more greatly reduced in the ARB+ HCTZ group than in the ACEs+HCTZ group.
All the BMI and WAIST/HIP ratio observed after the treatment in ARBs+HCTZ and ACE+HCTZ combination group there was no significant changes found in BMI and WAIST/HIP ratio. Various studies suggest high BMI as an important risk factor for different cardiac diseases. The Scandinavian Simvastation Survival Studies (SSSS) groups found BMI>22 Kg/m2 to be associated with many risk factors. The mean BMI of the patients in our study group (27.22, 26.71 kg/m2). satisfies the study (SSSS et al 1994) group criteria.
Lipid level was not changed significantly with the combination of an ARB and a thiazide diuretic and ACE with diuretic combination (Ishimitsu et al 2009). In the present study, none of the lipid parameters were changed significantly, although minimal change was suggested.
Overall, there were no unfavorable effects of ARB+ HCTZ and ACEs+HCTZ on lipid metabolism in this study.
In our study we observed that the use of the ARB+HCTZ and ACEs+HCTZ combination did not change parameters of either lipids metabolism or BMI, thus having a neutral metabolic profi le even when used in the long term. But there is significant changes were found in systolic blood pressure and diastolic blood pressure. The results of this study demonstrated that the ARB+HCTZ combination was significantly more effective at reducing SBP than ACEs+HCTZ combination. Based on these results we can conclude that this therapeutic modality is safe and adequate for the treatment of hypertension in patients with metabolic syndrome, diabetes mellitus and dyslipidemias.
There are some limitations to this study for interpretation. First, considering the number of t tests, there is a possibility that this study may have included a false positive result in the laboratory values. Second, the number of the patients may not have been enough, especially for the values with large variances to detect the difference between the 2 groups. Third, the data from the patients in this study were adjusted for sex, age, SBP, and lipid level at randomization.
Conclusion
The findings from this study suggest that ARB+HCTZ was more efficacious in decreasing SBP than was ACEs+HCTZ in the management of essential hypertension. Analysis of pooled data therefore demonstrates that, in patients with moderate-to-severe hypertension, ARB+HCTZ provides superior efficacy to ACE+HCTZ, with a high rate of BP goal achievement and similar levels of tolerability. These findings support the use of long-acting ARB+HCTZ such Telmisartan + hctz or olmesartan +hctz in combination provide improved BP control in the more severely hypertensive patient. Unfavorable effects on lipid metabolism were not observed with either combination therapy.
Summary
During the last decade, the hypertension disorder has progressively become a major worldwide public health problem, because of its association with increased risk of diabetes mellitus, atherosclerotic cardiovascular disease which may lead to morbidity and mortality.
The present study was conducted at Shri Mahant Indiresh Hospital Dehradun, Uttrakhand with a primary objective compared the efficacious and effect on lipid metabolism and blood pressure between ARBs+HCTZ and ACEs+HCTZ combination in patient who are suffering for hypertension.
From march 2013 to august 2013 a total of 100 patient. Out of which 95 patient completed 12 weeks of follow up. A total of 54 subjects randomly received treatments with ARBs+HCTZ out of 95 patients while 41 subjects received ACEs+HCTZ. The patients were diagnosed and prescribed medicines by a qualified physician. The data reported in this study is from the observation recorded in the individual prescription of individual patient, who met the inclusion criteria for being enrolled in the present study. Five patients excluded from this study due to lack of sufficient data.
At the start of initial investigation demographic information was collected from all the patient in a personal interview, anthropometric and biochemical parameter including height, weight, BMI, waist, hip, waist to hip ratio, lipid profile (HDL, LDL, TG, TC, VIDL,TC/HDL, TG/HDL,LDL/HDL.) were assessed on 1st visit. All the patient were followed for a period of 12 weeks to assess the response of treatment of hypertension and other cardiovascular disorder.
The results of this study demonstrated that the ARB+HCTZ combination was significantly more effective at reducing SBP than ACEs+HCTZ combination after 12 weeks of therapy in stage1and 2 hypertensive patients. Responder rates for SBP < 140 mmHg were significantly greater at Week 2 in the combination group, suggesting a more rapid onset of action, and were numerically greater in the combination group at Weeks 6 and 8, with p-values nearing statistical significance. Both treatments were generally well tolerated. These results support the recommendations of the (JNC-7 2010), the (European Society of Hypertension 2003), and the Taiwan Society of Cardiology Guidelines for the Management of Hypertension, which advocate early initiation of combination treatment consisting of two anti-hypertensive drugs with complimentary mechanisms of action for stage 2 hypertension patients (Neutel et al 2005), ( Dahlof et al 2002).
Lipid level was not changed significantly with the combination of an ARB and a thiazide diuretic and ACE with diuretic combination (Ishimitsu et al 2009). In the present study, none of the lipid parameters were changed significantly, although minimal change was suggested.
Overall, there were no unfavorable effects of ARB+ HCTZ and ACEs+HCTZ on lipid metabolism in this study.
In our study we observed that the use of the ARB+HCTZ and ACEs+HCTZ combination did not change parameters of either lipids metabolism or BMI, thus having a neutral metabolic profile even when used in the long term. But there is significant changes were found in systolic blood pressure and diastolic blood pressure. The results of this study demonstrated that the ARB+HCTZ combination was significantly more effective at reducing SBP than ACEs+HCTZ combination. Based on these results we can conclude that this therapeutic modality is safe and adequate for the treatment of hypertension in patients with metabolic syndrome, diabetes mellitus and dyslipidemias.
References
1. Yaseem N, Verma Rk, Donepudi A. “Efficay and tolerability of anti hypertensive drug in patients with mild to moderate hypertension in a tersiary care hospital” Departement of clinical pharmacy osmania genral hospital Archive of applied science research,2011;3(1):436-443.
2. Harvey, B. Simon, MD, Editor−in−Chief, Well−Connected reports; Associate Professor of Medicine,Harvard Medical School; Physician, Massachusetts General Hospital. Nov.2009.
3. Raquel D and jafari M, “angiotensin 2-receptor antagonist”. Am J health-syst pharma 2000;57:1231-38.
4. GOODMAN & GILMAN'S THE PHARMACOLOGICAL BASIS OF THERAPEUTICS - 11th Ed. (2006)
5. Giles TD, Materson BJ, Cohn JN, Kostis JB. Definition and classification of hypertension: an update. J Clin Hypertens (Greenwich). 2009;11(11):611–614.
6. Duarte, JD; Cooper-Dehoff, RM (2010). "Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics". Expert review of cardiovascular therapy 8 (6): 793–802.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 1 Received On: 02/012/2014; Accepted On: 17/12/2014; Published On: 15/01/2014 How to cite this article: S Rajak, P Kothiyal, P Mathur, To study the Efficacy and Effects on Lipid Metabolism, anthropometry and blood pressure of Combination Treatment with ARB+HCTZ and ACEs+HCTZ in hypertensive patient, PharmaTutor, 2014, 2(1), 112-128 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









