 About Authors:
About Authors:
Kapil Sharma*1, Subhash Gupta 2
1 Yaresun Pharmaceutical Pvt. Ltd.,India.
2 Oasis test house ltd. Jaipur-302006, Rajasthan, India.
*pharma_kapil@rediffmail.com
ABSTRACT
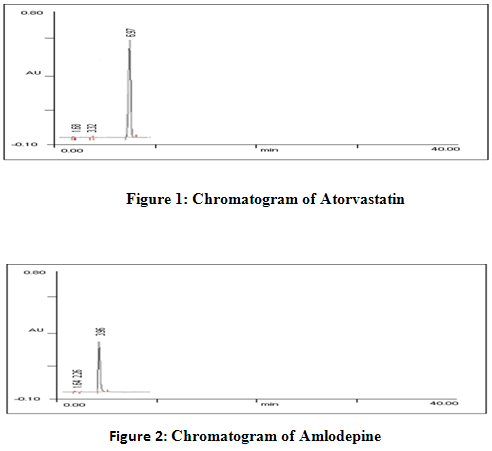
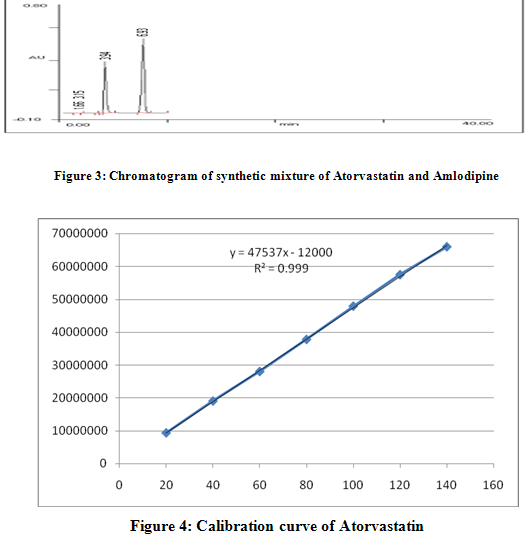
A method for simultaneous estimation of Atorvastatin (ATVS) and Amlodipine (AMLD) in raw material and in combined tablet dosage form has been developed. The method employs the application of RP-HPLC. Chromatgraphy was carried out on a nucleosil C-18,250 x 4.6 mm column using a mixture of phosphate buffer and methanol (50:50 v/v) as the mobile phase at a flow rate of 1.3 ml/min. Run time was 15 min. Detection was done at 245 nm and retention time of ATVS was 6.97 min and 3.96 min of AMLD.3 This method produced linear responses in the concentration range 20-140 µg/ml and 10-70 µg/ml for ATVS and AMLD respectively. The accuracy of the method was assessed by recovery studies and was found to be 100.54± 0.19 and 100.08±0.80 for ATVS and AMLD respectively.The procedure was successfully applied for the simultaneous determination of both drugs in laboratory prepared mixture of raw material and in market available tablet dosage form. Results of the analysis were validated statistically so that it can be used for routine analysis of ATVS and AMLD in combined tablet dosage form.4-6
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1408
INTRODUCTION
Atorvastatin calcium is chemically [R-(R, R*)]-2-(4-fluorophenyl)-β-δ-dihydroxy-5 (1-methylethyl)-3-phenyl-4 [(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1) trihydrate. It is inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, an early and rate limiting step in cholesterol biosynthesis. Amlodipine Besilate is chemically 3-Ethyl 5-methyl (4RS)-2-[(2-aminoethoxy) methyl]-4-(2-chlorophenyl)-6-methyl-1, 4- dihydropyridine-3, 5-dicarboxylate benzenesulphonate. Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle.2It is official in British Pharmacopoeia but not in I.P and USP.1Review of literature revels that there is not any method described for simultaneous estimation of Atorvastatin calcium and Amlodipine Besilate using a nucleosil C-18,250 x 4.6 mm column using a mixture of phosphate buffer and methanol (50:50 v/v) as the mobile phase at a flow rate of 1.3 ml/min.7-10The present paper describes simple, reproducible and sensitive RP-HPLC method for the determination of Atorvastatin Calcium and Amlodipine Besilate in raw materials and combined dosage form usinga nucleosil C-18,250 x 4.6 mm column using a mixture of phosphate buffer and methanol (50:50 v/v) as the mobile phase at a flow rate of 1.3 ml/min of 15 min run time on Detection at 245 nm.
MATERIAL AND METHOD
Instrument, reagents and chemicals
A shimadzu HPLC containing LC-10AT pump, variable wavelength programmable UV/Vis detector SPD-10AVP was used for the analysis. The method was carried out on a Nucleosil C-18 (250 x 4.6 MM) column as stationary phase and phosphate buffer with methanol (50:50 v/v) as mobile phase at a flow rate of 1.3 ml/min. A rheodyne injector with a 20 µl loop was used for the injection of samples. Gift sample of ATVS and AMLD was procured from Mankind Pharma, Ltd., Delhi. Tablets of 10 mg ATVS and 5 mg AMLD strength were procured from local pharmacy.
Preparation of mobile phase
Monobasic potassium phosphate was weighed (2.7 gm) and dissolved in 1000 ml HPLC grade water. pH of solution was adjusted with orthophosphoric acid and triethylamine to 3.5 ± 0.1 . This solution was mixed with methanol in the ratio of 50:50
Preparation of stock solution of Atorvastatin
Atorvastatin was weighed (100 mg) accurately and transferred to 100 ml volumetric flask. About 90 ml of HPLC grade methanol was added and sonicated to dissolve. The volume was made up to the mark with HPLC grade methanol. The final dilution contained about 1000μg/ml of Atorvastatin.
Preparation of stock solution of Amlodepine
Amlodepine was weighed (50 mg) accurately and transferred to 100 ml volumetric flask. About 90 ml of HPLC grade methanol was added and sonicated to dissolve. The volume was made up to the mark with HPLC grade methanol. The final dilution contained about 500μg/ml of Amlodepine.
Preparation of laboratory synthetic mixture of Atorvastatin and Amlodepine
Laboratory synthetic mixture of ATVS and AMLD were prepared in the ratio of 2:1 respectively. This decision is based on the combination of ATVS and AMLD available in the combined tablet dosage form. Laboratory synthetic mixture of ATVS and AMLD were prepared in the ratio of 20:10 respectively was prepared by dissolving 1 ml of ATVS stock solution and AMLD stock solution in to 50 ml HPLC grade methanol. Detection was done at 245 nm. The chromatogram of ATVS and AMLD are shown in figure 1 and 2. Chromatogram of synthetic mixture of Atorvastatin and Amlodepine is shown in figure 3.
Linearity and accuracy
Linearity and accuracy in the concentration range of 20-140 mg/ml for ATVS and 10-70 mg/ml for AMLD were examined employing intraday and interday studies for the determination of ATVS and AMLD. The results for intraday and interday experiments with a good correlation were obtained and evaluated statistically as demonstrated in table 1 and 2. Calibration curve is shown in figure 4 and 5.
Application of the proposed procedure for the estimation of Atorvastatin and Amlodepine in combined tablet dosage form
Twenty tablets were taken, and accurately weighed. The tablets were crushed to a fine powder. The powder sample equivalent to 10 mg of Atorvastatin and equivalent 5 mg of Amlodepine was transferred to a 50 ml volumetric flask and about 30 ml HPLC grade methanol was added and sonicated to dissolve. The volume was made up to the mark with HPLC grade methanol. This solution was filtered through whatman filter paper 42. This solution (5 ml) was diluted to 50 ml with HPLC grade methanol. The solutions were analyzed by multicomponent mode of analysis. As blank 50 % v/v methanol in distilled water was used. The solutions prepared were injected in five times into the HPLC system and the observations were recorded. A duplicate injection of the standard solution was also injected into the HPLC system and the chromatograms were recorded.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSIONS
A method for simultaneous estimation of Atorvastatin (ATVS) and Amlodipine (AMLD) in raw material and in combined tablet dosage form has been developed. The method employs the application of RP-HPLC. Chromatgraphy was carried out on a nucleosil C-18,250 x 4.6 mm column using a mixture of phosphate buffer and methanol (50:50 v/v) as the mobile phase at a flow rate of 1.3 ml/min. Run time was 15 min. Detection was done at 245 nm and retention time of ATVS was 6.97 min and 3.96 min of AMLD. This method produced linear responses in the concentration range 20-140 µg/ml and 10-70 µg/ml for ATVS and AMLD respectively. The accuracy of the method was assessed by recovery studies and was found to be 100.54± 0.19 and 100.08±0.80 for ATVS and AMLD respectively.The value of relative standard deviation in repeatability study was found 0.252 % for Atorvastatin and 0.383% for Amlodepine. In intra-day precision value of relative standard deviation was found 0.788 % for Atorvastatin and 0.037 % for Amlodepine. The value of relative standard deviation for inter-day precision was found 0.964 % for Atorvastatin and 0.157 % for Amlodepine. Hence the newly developed method can be used for routine analysis for the estimation of Atorvastatin and Amlodepine in raw material and in combined tablet dosage form.
ACKNOWLEDGEMENTS
The authors are thankful to Yaresun pharmaceutical Pvt. Ltd for providing the facilities to carry out the work. The authors are also thankful to vice president of oasis test house ltd, jaipur for providing working standard of Atorvastatin and Amlodepine.
REFERENCES
1. British Pharmacopoeia, department of health, Great Britain, Vol-1, 2003; 65, 124.
2. Rang H.P., Dale M.M., Ritter J.M., and Moore P. K., Pharmacology, 5th Edition, 2003, Churchill Living Stone, 345.
3. Snynder LR, Kirklend J J, Practical H.P.L.C. method development, 2nd ed., John Wiley and Sons, Inc: 2000; 2(1); 38-42.
4. Robert A. Nash, Alfred H. Wachter, Pharmaceutical process validation, Marcel Dekker, Inc., 2003, 507-22
5. Text on Validation of Analytical Procedures Q2A in; I.C.H. Harmonised Tripartite Guidelines; Oct. 1994.
6. Guidelines for the validation of analytical methods for active constituent, agriculture and veterinary chemical products, Australian Pesticides & Veterinary Medicines Authority, October 2004.
7. Malesuik MD, Cardoso SG, Bajerski L, Lanzanova FA. Determination of amlodipine in pharmaceutical dosage forms by liquid chromatography and ultraviolet spectrophotometry, Journal of AOAC International, 2006 Mar-Apr; 89(2): 359-64.
8. Rajeswari K Raja, et. al., RP-HPLC method for the simultaneous determination of Atorvastatin and Amlodipine in tablet dosage form, Indian Journal of Pharmaceutical Sciences, year 2006, volume 68, issue 2, page 275-277.
9. Manoj K, Shanmugapandiyan P, Anbazhagan S. RP HPLC method for simultaneous estimation of Atorvastatin and aspirin from capsule formulation. Indian Drugs, 2004; 41(5): 284-289.
10. Erturk S, Sevinc Aktas E., et. al, An HPLC method for the determination of Atorvastatin and its impurities in bulk drug and tablets, J Pharm Biomed Anal., 2003 Dec 4; 33(5):1017-23.
Table 1: Recovery study of Atorvastatin and Amlodepine
|
S.No |
Drug |
Conc. before spiking (mg/ml) |
Reference Std. added (mg/ml) |
Conc. after spiking (mg/ml) |
percent Recovery |
Mean percent Recovery |
|
1 |
Atorvastatin |
59.16 |
40.10 |
100.06 |
100.81 |
100.54 ± 0.19 |
|
59.16 |
60.05 |
119.76 |
100.46 |
|||
|
59.16 |
80.10 |
139.76 |
100.36 |
|||
|
2 |
Amlodepine |
30.12 |
19.32 |
49.34 |
99.79 |
100.08 ± 0.80 |
|
30.12 |
29.40 |
59.12 |
99.33 |
|||
|
30.12 |
39.31 |
70.21 |
101.12 |
Table 2: Summary of Validation Parameters
|
PARAMETER |
OBSERVATION |
|
|
Atorvastatin |
Amlodepine |
|
|
Linearity (Correlation coefficient r2) |
0.999 |
0.998 |
|
Accuracy (% Recovery) |
100.54 ± 0.19 |
100.08 ± 0.80 |
|
Precision RSD Repeatability (n= 6) Intra-day (n=3) Inter-day (days=3) |
0.252 0.788 0.964 |
0.383 0.037 0.157 |

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









