 About Authors:
About Authors:
C.P.Meher
Asst. Professor
Maheshwara Institute of Pharmacy,
Chitkul, Patancheru, Medak, A.P
chaitanyameher84@gmail.com
ABSTRACT
Pyrazole, a 5-membered heterocyclic diazole alkaloid composed of three carbon atoms and two nitrogen atoms in adjacent positions, is a prevalent scaffold in drug discovery programs. The aim of this review is to provide an overview of diverse pharmacological activities of pyrazole moiety.This review highlighted recent reports of antimicrobial, anticancer, ACE inhibitory, antiviral as well as anti-inflammatory activities of pyrazole. The purpose of this review was to collate literature work reported by researchers on pyrazole for their varoius pharmacological activities and also reported recent efforts made on this moiety.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1559
INTRODUCTION
The term Pyrazole was given by Ludwig Knorr in 1883. Pyrazole is the organic compound with the formula C3H3N2H. Pyrazole refers to the class of simple aromatic ring organic compounds of the heterocyclic series. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen centres. Pyrazoles are also the class of compounds that have the ring C3N2 with adjacent nitrogen centres. Notable drug that is a pyrazole is Celebrex. Derivatives of pyrazole are used for their analgesic, anti-inflammatory, antipyretic, antiarrhythmic, tranquilizing, muscle relaxing, psychoanaleptic, anticonvulsant, monoamineoxidase inhibiting, antidiabetic and antibacterial activities. The pyrazole ring is present as the core in a variety of leading nonsteroidal anti-inflammatory drugs (NSAIDs) and antihypertensive drugs. They have also found use as bifunctional ligands for metal catalysis, and in various building blocks for pharmaceutical and agricultural research. Being so composed and having pharmacological effects on humans, they are classified as alkaloids, although they are rare in nature. In 1959, the first natural pyrazole, 1-pyrazolyl-alanine, was isolated from seeds of watermelons .Pyrazole derivatives have a long history of application in agrochemicals and pharmaceutical industry as herbicides and active pharmaceuticals. The recent success of pyrazole COX-2 inhibitor has further highlighted the importance of these heterocyclic rings in medicinal chemistry. A systematic investigation of this class of heterocyclic lead revealed that pyrazole containing pharmacoactive agents play important role in medicinal chemistry. The prevalence of pyrazole cores in biologically active molecules has stimulated the need for elegant and efficient ways to make these heterocyclic lead. Pyrazoles have been the recent target of numerous methodologies, mostly due to their prevalence as scaffolds in drug discovery programs and synthesis in particular of bioactive compounds and reactions in different media. The pyrazole ring is present as the core in a variety of leading drugs such as Celebrex, Viagra or Rimonabant. They have also found use as bifunctional ligands for metal catalysis, and in various building blocks for pharmaceutical and agricultural research. The pyrazole compounds are not known to occur in nature; they are usually prepared by the reaction of hydrazines with 1,3-diketones. Many synthetic pyrazole compounds are of importance as dyes and medicinals. Among them are: antipyrine, used as an analgesic and febrifuge; tartrazine, most commonly used as a yellow dye for food; phenylbutazone (Butazolidin), an anti-inflammatory drug used in treatment of arthritis; and a series of dyes used as sensitizing agents in colour photography. Some properties of pyrazole nucleus are given in table-1.
[adsense:468x15:2204050025]
Table-1
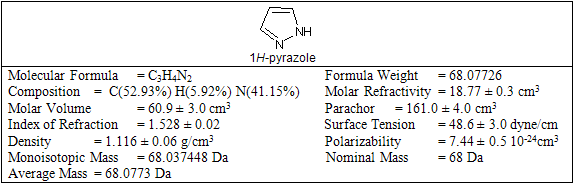
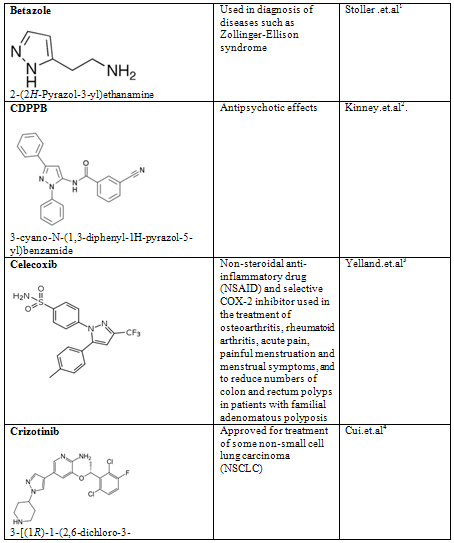
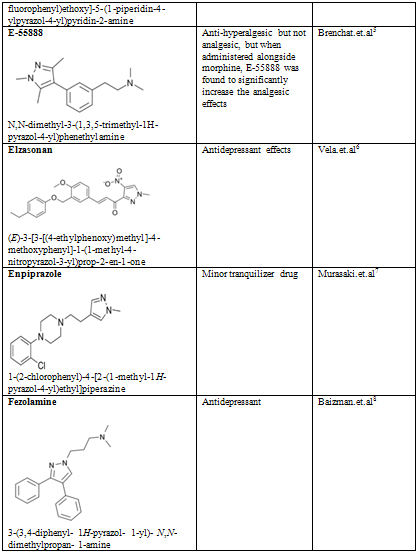
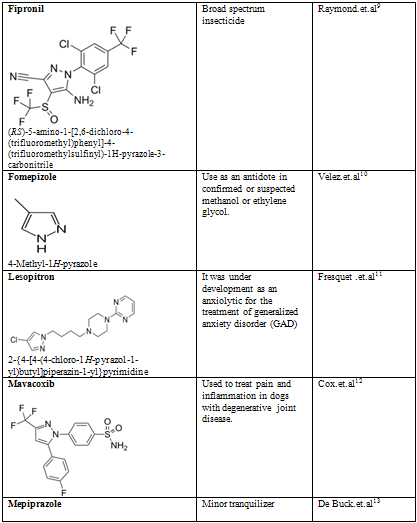
Pyrazoles are stable compounds with relatively high boiling points. It is a colorless compound with a peculiarly penetrating sweetish smell. The introduction of substituents in the 3-, 4-, and 5-positions causes an increase in the boiling point, which is expected with increased molecular weight, but the boiling point falls sharply with substitution in the 1-position. Similarly, 1-substituted pyrazoles melt at temperatures significantly lower than the corresponding compounds with free N-H groups. Both of these anomalies are due to the particularly strong association of pyrazoles unsubstituted in position 1, as proved by cryoscopic determination, spectral studies, dipole moment measurement, and infrared spectroscopy. Different biological activity possess by the pyrazole derivative are given below in table-2
Table-2
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
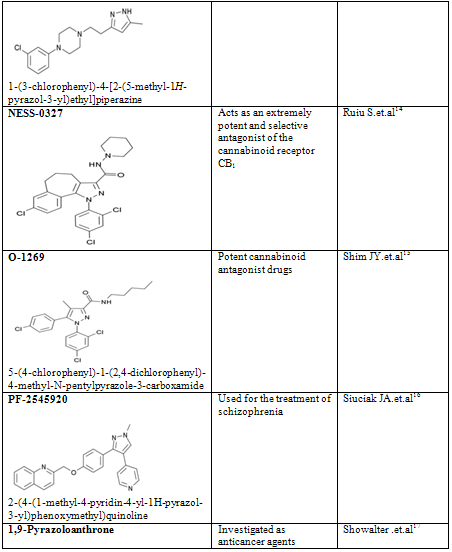
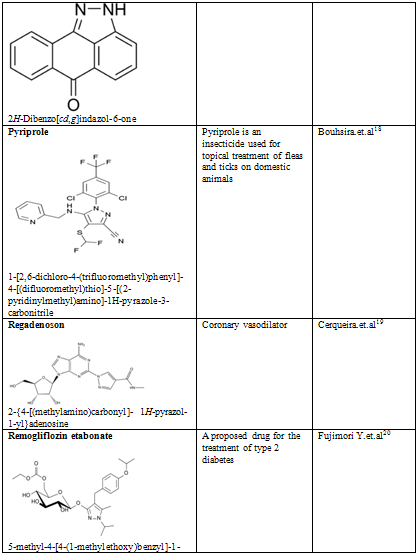
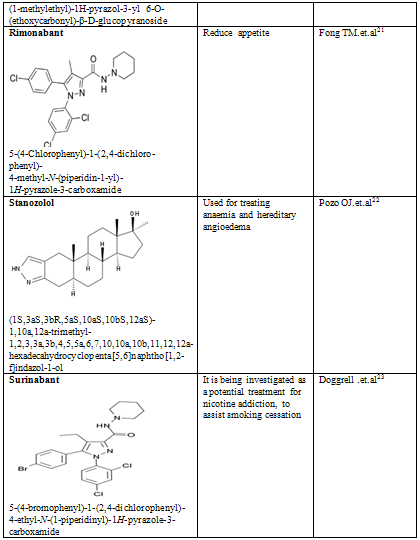
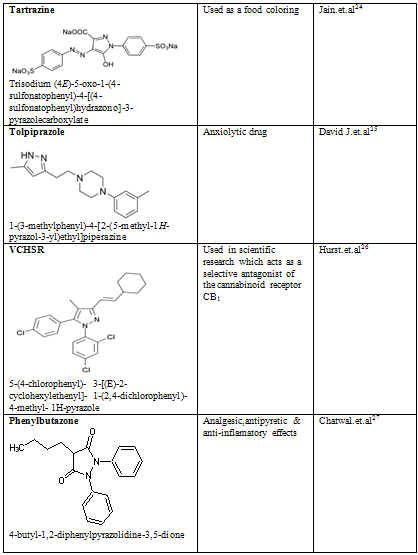
CONCLUSION
Pyrazole is a unique template that is associated with several biological activities. The review has presented comprehensive details of pyrazole derivatives. Several economical and social merits have been prospected for compounds with effects like antiinflammation, anxiolytic, anaemia and hereditary angioedema, appetite, Antipsychotic and others. Pyrazoles are an important class of compounds for new drug development that attracted much attention. Several pyrazole derivatives have been synthesized as target structures and evaluated for their biological activities. Pyrazole used as antioxidants in motor fuels, medicine, and development of cine-film. Besides the traditional interest in pyrazole derivatives which have been the basis of numerous dyes and drugs, a number of pyrazole anesthetics have appeared. Recent studies have established that pyrazolines are intermediates in the synthesis of cyclopropanes; they act as effective chemical bleaching agents, and as a constituent of luminescent and fluorescent substances.
REFERENCES
1. Stoller JL Holubitsky IB Harrison RC Munro AI (1970). "Complications of the histalog test of gastric acid secretion". Digestive Diseases and Sciences 15 (7): 647–651.
2. Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL (April 2005). "A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models". The Journal of Pharmacology and Experimental Therapeutics 313 (1): 199–206.
3. Yelland MJ, Nikles CJ, McNairn N, Del Mar CB, Schluter PJ, Brown RM (2007). "Celecoxib compared with sustained-release paracetamol for osteoarthritis: a series of n-of-1 trials". Rheumatology 46 (1): 135–40.
4. Cui, J. J.; Tran-Dubé, M.; Shen, H.; Nambu, M.; Kung, P. P.; Pairish, M.; Jia, L.; Meng, J. et al. (2011). "Structure Based Drug Design of Crizotinib (PF-02341066), a Potent and Selective Dual Inhibitor of Mesenchymal–Epithelial Transition Factor (c-MET) Kinase and Anaplastic Lymphoma Kinase (ALK)". Journal of Medicinal Chemistry 54 (18): 6342–6363.
5. Brenchat A, Romero L, García M, Pujol M, Burgueño J, Torrens A, Hamon M, Baeyens JM, Buschmann H, Zamanillo D, Vela JM (February 2009). "5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice". Pain 141 (3): 239–47.
6. osé Miguel Vela; Helmut Buschmann; Jörg Holenz; Antonio Párraga; Antoni Torrens (2007). Antidepressants, Antipsychotics, Anxiolytics: From Chemistry and Pharmacology to Clinical Application
7. Murasaki M, Hara T, Oguchi T, Inami M, Ikeda Y (September 1976). "Action of enpiprazole on emotional behavior induced by hypothalamic stimulation in rats and cats". Psychopharmacology 49 (3): 271–4
8. Baizman ER, Ezrin AM, Ferrari RA, Luttinger D (October 1987). "Pharmacologic profile of fezolamine fumarate: a nontricyclic antidepressant in animal models". The Journal of Pharmacology and Experimental Therapeutics 243 (1): 40–54.
9. Raymond-Delpech V, Matsuda K, Sattelle BM, Rauh JJ, Sattelle DB (2005) Ion channels: molecular targets of neuroactive insecticides. Invert Neurosci: 1-15.
10. Velez LI, Shepherd G, Lee YC, Keyes DC (September 2007). "Ethylene glycol ingestion treated only with fomepizole". J Med Toxicol 3 (3): 125–8.
11. Fresquet A, Sust M, Lloret A, et al. (February 2000). "Efficacy and safety of lesopitron in outpatients with generalized anxiety disorder". The Annals of Pharmacotherapy 34 (2): 147–53
12. Cox, S. R.; Lesman, S. P.; Boucher, J. F.; Krautmann, M. J.; Hummel, B. D.; Savides, M.; Marsh, S.; Fielder, A. et al. (2010). "The pharmacokinetics of mavacoxib, a long-acting COX-2 inhibitor, in young adult laboratory dogs". Journal of Veterinary Pharmacology and Therapeutics 33 (5): 461–70.
13. De Buck R, Van Durme R, Pelc I (May 1975). "[A controlled double-blind crossover study of the efficacy of mepiprazol (EMD 16.923) and of diazepam in the treatment of neurotic disorders]" (in French). Acta Psychiatrica Belgica 75 (3): 320–33.
14. Ruiu S, Pinna GA, Marchese G, Mussinu JM, Saba P, Tambaro S, Casti P, Vargiu R, Pani L. Synthesis and characterization of NESS 0327: a novel putative antagonist of the CB1 cannabinoid receptor. Journal of Pharmacology and Experimental Therapeutics. 2003 Jul;306(1):363-70
15. Shim JY, Welsh WJ, Cartier E, Edwards JL, Howlett AC. Molecular interaction of the antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1- (2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide with the CB1 cannabinoid receptor. Journal of Medicinal Chemistry. 2002 Mar 28;45(7):1447-59
16. Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L, Shrikhande A, Wong S, Menniti FS, Schmidt CJ. Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology. 2006 Aug;51(2):386-96.
17. Showalter HD, Johnson JL, Werbel LM, Leopold WR, Jackson RC, Elslager EF (1984). "5-[(Aminoalkyl)amino]-substituted anthra[1,9-cd]pyrazol-6(2H)-ones as novel anticancer agents. Synthesis and biological evaluation". J. Med. Chem. 27 (3): 253–5.
18. Bouhsira E, Fysikopoulos A, Franc M. Efficacy of fipronil-(S)-methoprene, metaflumizone combined with amitraz, and pyriprole commercial spot-on products in preventing Culex pipiens pipiens from feeding on dogs. Veterinary Record. 2009 Aug 1;165(5):135-7
19. Cerqueira MD (July 2004). "The future of pharmacologic stress: selective A2A adenosine receptor agonists". Am. J. Cardiol. 94 (2A): 33D–40D; discussion 40D–42D
20. Fujimori Y, Katsuno K, Nakashima I, Ishikawa-Takemura Y, Fujikura H, Isaji M (June 2008). "Remogliflozin etabonate, in a Novel Category of Selective Low-Affinity / High-Capacity Sodium Glucose Cotransporter (SGLT2) Inhibitors, Exhibits Antidiabetic Efficacy in Rodent Models". J. Pharmacol. Exp. Ther. 327 (1): 268–276
21. Fong TM, Heymsfield SB (September 2009). "Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions". Int J Obes (Lond) 33 (9): 947–55
22. Pozo OJ, Van Eenoo P, Deventer K et al. Detection and structural investigation of metabolites of stanozolol in human urine by liquid chromatography tandem mass spectrometry. Steroids 74: 837-852, 2009.
23. Doggrell SA. Will the new CB1 cannabinoid receptor antagonist SR-147778 have advantages over rimonabant? Expert Opinion on Investigational Drugs. 2005 Mar;14(3):339-42
24. Jain, Rajeev; Bhargava, Meenakshi; Sharma, Nidhi (2003). "Electrochemical Studies on a Pharmaceutical Azo Dye: Tartrazine". Industrial & Engineering Chemistry Research 42 (2): 243.
25. David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC.
26. Hurst, D; Umejiego, U; Lynch, D; Seltzman, H; Hyatt, S; Roche, M; McAllister, S; Fleischer, D et al. (2006). "Biarylpyrazole inverse agonists at the cannabinoid CB1 receptor: importance of the C-3 carboxamide oxygen/lysine3.28(192) interaction". Journal of Medical Chemistry 49 (20): 5969–87.
27. Pharmaceutical organic chemistry-II,chatwal.G.R, P-2.4.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









