 About Authors
About Authors
Deepak Kumar Shukla*, Nazia Shahid, Vikas Kumar Alaria
Rajasthan Pharmacy College, Bhankrota, Jaipur (Raj.)
*deepakshukla.pharma@gmail.com
Abstract
Over the past several years spectroscopy has become the preeminent technique for determining the structure of organic compounds. The study of recoilless nuclear resonant absorption or fluorescence is more commonly known as Mossbauer spectroscopy. From its first origins in 1957, it has grown rapidly to become one of the most important research methods in solid-state physics and chemistry. Mossbauer spectroscopy uses the nuclear properties to get information regarding the environment surrounding the nucleus. This technique is now valid application in diverse fields, such as solid state physics, metallurgy, chemistry and biochemistry. For example, it is possible to use this method for estimating the iron or tin content in ores, alloys and wasters in a non-destructive manner to concentration down to 0.03 percent in a short time of the order of 10 minutes. The technique can also detect the relative percentage of different charged states of the same atom, for example fe2+ and Fe3+ present in the material. This is somewhat difficult to get from any other technique.
[adsense:336x280:8701650588]
The Mossbauer effect (ME) is based on the recoil-free-γ-rays resonance absorption phenomenan. Its unique feature is the production of highly monochromatic electromagnetic radiation so that it can be used for resolving minute energy perturbations. The application of ME arises from its ability to detect the small variations in the energy of interaction between the nucleus and extra-nuclear electrons which prior to the discovery of this effect were regarded hardly possible to observe.
REFERENCE ID: PHARMATUTOR-ART-1720
Fundamental Principles of Mössbauer Spectroscopy
The Mössbauer Effect
Nuclei in atoms undergo a variety of energy level transitions, often associated with the emission or absorption of a gamma ray. These energy levels are influenced by their surrounding environment, both electronic and magnetic, which can change or split these energy levels. These changes in the energy levels can provide information about the atom's local environment within a system and ought to be observed using resonance-fluorescence. There are, however, two major obstacles in obtaining this information: the 'hyperfine' interactions between the nucleus and its environment are extremely small, and the recoil of the nucleus as the gamma-ray is emitted or absorbed prevents resonance.
In a free nucleus during emission or absorption of a gamma ray it recoils due to conservation of momentum, just like a gun recoils when firing a bullet, with a recoil energy ER. This recoil is shown in Fig1. The emitted gamma ray has ER less energy than the nuclear transition but to be resonantly absorbed it must be ER greater than the transition energy due to the recoil of the absorbing nucleus. To achieve resonance the loss of the recoil energy must be overcome in some way.

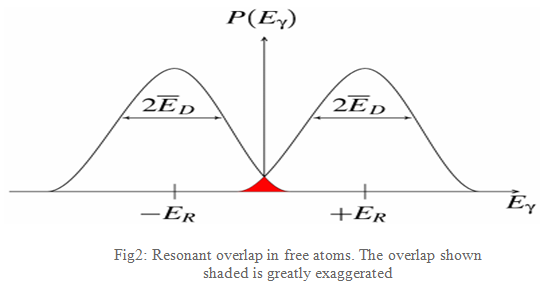
As the atoms will be moving due to random thermal motion the gamma-ray energy has a spread of values ED caused by the Doppler effect. This produces a gamma-ray energy profile as shown in Fig2. To produce a resonant signal the two energies need to overlap and this is shown in the red-shaded area. This area is shown exaggerated as in reality it is extremely small, a millionth or less of the gamma-rays are in this region, and impractical as a technique.
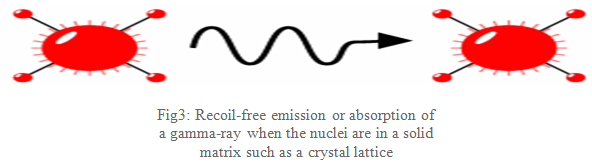
What Mössbauer discovered is that when the atoms are within a solid matrix the effective mass of the nucleus is very much greater. The recoiling mass is now effectively the mass of the whole system, making ER and ED very small. If the gamma-ray energy is small enough the recoil of the nucleus is too low to be transmitted as a phonon (vibration in the crystal lattice) and so the whole system recoils, making the recoil energy practically zero: a recoil-free event. In this situation, as shown in Fig3, if the emitting and absorbing nuclei are in a solid matrix the emitted and absorbed gamma-ray is the same energy: resonance!
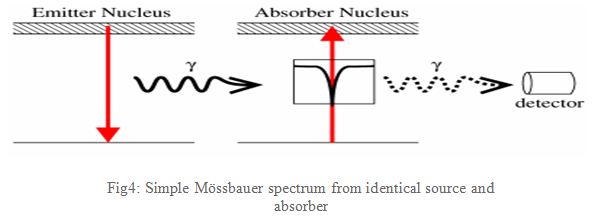
If emitting and absorbing nuclei are in identical, cubic environments then the transition energies are identical and this produces a spectrum as shown in Fig4: a single absorption line.
The Mössbauer effect as generally applied to the study of minerals relies on the fact that 57Fe, which is a decay product of 57Co, is unstable. 57Fe decays by giving off a gamma ray (γ-ray), along with other types of energy. Figure 1 shows the nuclear decay scheme for 57Co → 57Fe and various backscattering processes for 57Fe that can follow resonant absorption of an incident gamma photon, modified from DeGrave et al. (2005) and Dyar et al. (2006). If a nucleus gives off radiation or any other form of energy (in this case, in the form of a γ-ray), the nucleus must recoil (or move) with an equal and opposite momentum to preserve its energy (E), in the same way that a gun (by analogy, the nucleus) recoils when a bullet (the γ-ray) is fired out of it. We des cribe this general case in terms of energy by saying that:
|
Eγ-ray emission = Etransition - ER, |
Where, Eγ-ray emission = the energy of the emitted γ-ray Etransition = the energy of the nuclear transition |

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Mössbauer Spectroscopy Instrumentation
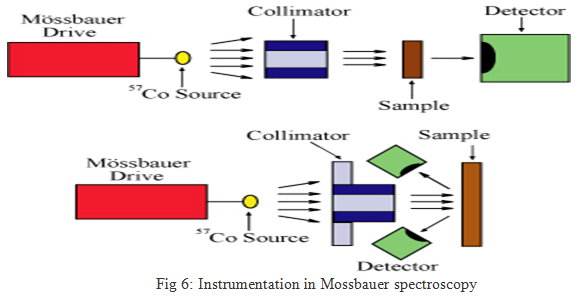
The basic elements of a Mössbauer spectrometer are a source, sample, detector, and a drive to move the source or absorber. Most commonly, this is done by moving the source toward and away from the sample, while varying velocity linearly with time. For example, for 57Fe, moving the source at a velocity of 1 mm/sec toward the sample increases the energy of the emitted photons by about ten natural linewidths. For simplicity, "mm/sec" is the conventional "energy" unit in Mössbauer spectroscopy. It is also possible to leave the source stationary and oscillate the sample, as is done with synchrotron Mössbauer. The location of the detector relative to the source and the sample defines the geometry of the experiment (Figure 5); most commonly, either transmission or backscatter modes are used.
Typical Method
In its most common form, Mössbauer absorption spectroscopy, a solid sample is exposed to a beam of gamma radiation, and a detector measures the intensity of the beam transmitted through the sample. The atoms in the source emitting the gamma rays must be of the same isotope as the atoms in the sample absorbing them.
If the emitting and absorbing nuclei were in identical chemical environments, the nuclear transition energies would be exactly equal and resonant absorption would be observed with both materials at rest. The difference in chemical environments, however, causes the nuclear energy levels to shift in a few different ways, as described below. Although these energy shifts are tiny (often less than a micro-electronvolt), the extremely narrow spectral linewidths of gamma rays for some radionuclides make the small energy shifts correspond to large changes in absorbance. To bring the two nuclei back into resonance it is necessary to change the energy of the gamma ray slightly, and in practice this is always done using the Doppler effect.
During Mössbauer absorption spectroscopy, the source is accelerated through a range of velocities using a linear motor to produce a Doppler effect and scan the gamma ray energy through a given range. A typical range of velocities for 57Fe, for example, may be ±11 mm/s (1 mm/s = 48.075 neV).[2][3]
In the resulting spectra, gamma ray intensity is plotted as a function of the source velocity. At velocities corresponding to the resonant energy levels of the sample, a fraction of the gamma rays are absorbed, resulting in a drop in the measured intensity and a corresponding dip in the spectrum. The number, positions, and intensities of the dips (also called peaks; dips in transmitted intensity are peaks in absorbance) provide information about the chemical environment of the absorbing nuclei and can be used to characterize the sample.
Selecting a suitable source
Mössbauer spectroscopy is limited by the need for a suitable gamma-ray source. Usually, this consists of a radioactive parent that decays to the desired isotope. For example, the source for 57Fe consists of 57Co, which decays by electron capture to an excited state of 57Fe, then subsequently decays to a ground state emitting the desired gamma-ray. The radioactive cobalt is prepared on a foil, often of rhodium.[4] Ideally the parent isotope will have a sufficiently long half-life to remain useful, but will also have a sufficient decay rate to supply the required intensity of radiation. Also, the gamma-ray energy should be relatively low, otherwise the system will have a low recoil-free fraction resulting in a poor signal-to-noise ratio and requiring long collection times. The periodic table below indicates those elements having an isotope suitable for Mössbauer spectroscopy. Of these, 57Fe is by far the most common element studied using the technique, although 129I, 119Sn, and 121Sb are also frequently studied.

Strengths and Limitations of Mössbauer Spectroscopy
Strengths
Along with wet chemistry, Mössbauer spectroscopy remains the "gold standard" for quantitative determination of the valence state of iron in minerals and identification of various iron oxides. It is also well-suited for determination of the coordination number of Fe atoms.
Limitations
The biggest limitation of the Mössbauer is that it is inherently a bulk technique; it uses powders spread thinly across an absorber to get optimal experimental conditions. In recent years, improvements in electronics and detectors have made it possible to run very small samples (1-5 mg). Another approach to this problem is the Mössbauer milliprobe developed by Catherine McCammon at Bayreuth (e.g. McCammon, 1994). This modification, which uses a lead plate to restrict gamma rays to a small diameter (~100 μm), can be used to study single grains in thin sections or single crystals.
The vast majority of rock-forming minerals on Earth contain Fe2+ in octahedral coordination, and thus have very similar Mössbauer parameters. For example, pyroxene, amphibole, and mica spectra are all nearly indistinguishable. Furthermore, most minerals exhibit a range of Mössbauer parameters as a function of cation substitution. Finally, the parameters vary as a function of temperature, and the magnitude of that variation is distinctive to each mineral composition. For these reasons, Mössbauer spectroscopy is not ideally suited to mineral identification (except for iron oxides, where magnetic properties can be extremely diagnostic) and is typically not used for this purpose (though it has been pressed into such service in extraterrestrial applications).
Applications
1. Analytical Chemistry:- The technique is being used to determine the iron and tin content in ores, alloys and industrial wastes. It is possible to detect the presence of small amount of iron in complex system such as iron rust and asbestos. Fauther the concentration of different charge states of the same atoms like Fe2+ and Fe3+, present in a material like Fe3O4 can be readily estimated as these two states have different isomer shift, quadrupole splitting and nuclear Zeeman splitting.
2. Metallurgy:- The technique can be used to study the compositional inhomogeneity in alloys, corrosions mechanism, thermal decomposition processes, etc., at the atomic level.
3. Electronic structure and bonding:- The isomer shift and quadrupole splitting data provide information about the electronic state and the character of covalent bonding including the electron acceptor and donor properties of the atom. This also makes the computation of the electron charge cloud distribution in the atom.
References
[1] Mössbauer Spectroscopy M. Darby Dyar, Department of Astronomy, Mount Holyoke College Availible on UR:serc.carleton.edu/research_education/geochemsheets/techniques/mossbauer.html]
[2] Gütlich, J.M.; The Principle of the Mössbauer Effect and Basic Concepts of Mössbauer Spectrometry URL: pecbip2.univ-lemans.fr/webibame/
[3] Mössbauer Spectroscopy Group, Royal Society of Chemistry (RSC) website, Introduction to Mössbauer Spectroscopy Part 1 Accessed June 3, 2010 URL:rsc.org/membership/networking/interestgroups/mossbauerspect/intropart1.asp
[4] Critical Review on Chemical Applications of Mossbauer Spectroscopy E. KUZMANN, S. NAGY, AND A. VÉRTES, Laboratory of Nuclear Techniques in Structural Chemistry, Hungarian Academy of Sciences, Budapest, Hungary; 2Department of Nuclear Chemistry, Eötvös Loránd University, Budapest, Hungary URL:pac.iupac.org/publications/pac/pdf/2003/pdf/7506x0801.pdf
[5] Hollas J.M, “Modern Spectroscopy”, Fourth edition, Jhon Wiley & Sons Ltd. England, Page No: 289-317.
[6] Chatwal G.R.,Anand S.K, “Instrumental Methods of Chemical Analysis”, Fifth Edition 2002, Himalaya Publishing House Mumbai, Page No:-2.418-2.427
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









