{ DOWNLOAD AS PDF }
About Authors:
1Neeta Chaudhary, 2Dr. Shalini Tripathi
1Research student of Rameshwaram Institute of Technology and Management, Sitapur Road, Lucknow (U.P.)
2Professor of Rameshwaram Institute of Technology and Management, Sitapur Road,
Lucknow (U.P.)
neetakhanna70@gmail.com
Abstract
Trifolium pratense L. is commonly known as red clover. Red clover acting as a diuretic, expectorant and improving circulation. Trifolium pratense isoflavones, plant based chemicals that have shown potential in the treatment of a number of conditions associated with menopause, such as hot flashes, cardiovascular health, and osteoporosis. Literature review revealed different pharmacological activities of Trifolium pratense like Biological estimation of oestrogenic activity An ovariectomized rat model to determine the estrogenic and antiestrogenic activity, Antioxidant Profile, isoflavone biochanin A inhibits aromatase activity and expression, phase IIclinical extract possesses opiate activity, Determination in vivo and in vitro anti-inflammatory activity of red clover.
REFERENCE ID: PHARMATUTOR-ART-2122
GENERAL INTRODUCTION
Trifolium pratense L. is commonly known as red clover (Piersenet al., 2004). In Chinese medicine, Trifolium pratense was used as a sedative and for pain and other symptoms related to cancer, rheumatism, and gout (Duke and Ayensu, 1985). It has also been used for over 100 years in Europe and America to treat whooping cough, pain, and as a sedative and an expectorant (Hamrick JL et al., 1979).It can be grown in a wide range of soil types, pH levels and environmental conditions (Smith et al. 1985) and gives satisfactory yield in areas that are not suitable for growing alfalfa because the soils are too wet and/or too acid. Red clover is a significant forage legume grown in eastern Canada and the north eastern United States. Through symbiotic nitrogen fixation, red clover also provides nitrogen for soils, companion crops and subsequent crops. The range of fixed nitrogen was estimated to be from 125 to 220 kg ha·1year·1
(LaRue & Patterson ,1981; Rohweder et al. 1977). These qualities have made red clover useful for hay, silage, pasture, intercropping and green manure in several countries (Smith et al. 1985).
CLASSIFICATION
Trifoliumpratense
Kingdom: Plantae
Subkingdom: Tracheobionta
Superdivision: Spermatophyta
Division: Magnoliophyta
Class: Magnoliopsida
Subclass: Rosidae
Order: Fabales
Family: Fabaceae
Genus: Trifolium L
(plants.usda.gov/java/profile?symbol=trpr2)
PLANT PROFILE

Other common name(s) : Beebread, cow clover, meadow clover, purple clover, trefoil, wild clover
Scientific/medical name(s):Trifolium pratense
Plant Parts Used:The dried and the fresh flower heads .
Active Components: Red clover is one of the richest sources of isoflavones, water-soluble chemicals that act like estrogens and are found in many plants.
Looks like: The plant is a perennial herb 15 to 40 cm high. The red flowers are the source of medicinal properties.
Where it’s grown: Commonly grows wild in meadows throughout Europe and Asia, and has now been naturalized in many other parts of the world.
(akuna.net/picture/Canada_WEB/PDFs/RED%20CLOVER.pdf)
GEOGRAPHICAL DISTRIBUTION
Red clover is a relatively short-lived perennial, originating from southeastern Eurasia; it is widely distributed across the temperate zones of the world (Taylor & Quesenberry, 1996). Red clover is considered to be native to Sweden and was scientifically named by Carl Linnaeus in 1753, but was first described in Sweden in 1658 by Rudbeck in the Catalogus plantarum (Nordstedt, 1920). Cultivation involving ploughing meadows and re-sowing with forage species in planned crop rotations began in the late 18th century in the southern part of Sweden; the same system came into use in the north a century later (Hagsand & Wik, 1968). Red clover lives in symbiosis with Rhizobium leguminosarum, a nitrogen-fixing, nodule-forming bacterium. In a T. pratense monoculture in Sweden, the amount of fixed nitrogen (N) varies between 2.2 and 8.5 g N m-2 year-1 over four years (Carlsson et al., 2005).
MORPHOLOGICAL DESCRIPTION
This perennial plant is ½–2' tall, branching occasionally. The hairy stems are sprawling or erect. The alternate compound leaves are trifoliate. The lower compound leaves have long hairy petioles, while the upper leaves have short petioles or they are sessile. The leaflets are up to 2" long and ¾" across. They are oval-ovate or slightly obviate; sometimes they are a little broader below the middle. Their margins are smooth and ciliate and their tips are blunt. Toward the middle of the upper surface of each leaflet, there is usually a chevron that is white or light green. The leaflets are sessile and lack petioles of their own. At the base of each compound leaf, there is a pair of ovate stipules up to ½" long. The upper stems terminate in flower heads that are spheroid or ovoid. Usually there are 1-3 leaflets immediately beneath each flower head, as well as several green bracts with tips that abruptly taper to a slender tip. Each flower head is about 1" across and consists of numerous flowers. These flowers are sessile, tubular-shaped, and spread outward in different directions. Each flower has 5 narrow petals that are pink or purplish pink, becoming light pink or white toward the base of the flower head; a rare form of this species with white petals also exists. The upper petal is slightly longer than the lower petals. The light green calyx of each flower has 5 slender teeth and it is usually hairy.
The blooming period usually occurs from late spring to mid-summer and lasts about 1-2 months. However, a few plants may bloom later in the summer or fall. The flowers have a mild honey-like fragrance, while the foliage, when it exists in abundance, produces a distinctive clover-like aroma that is quite pleasant. Each flower is replaced by a small seedpod containing 1 or 2 heart-shaped seeds. The root system consists of a taproot and produces rhizomes.
(illinoiswildflowers.info/weeds/plants/red_clover.htm)
MICROSCOPICAL DESCRIPTION:
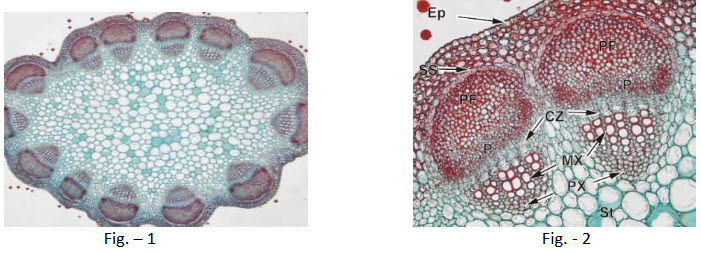
Trifolium (red clover) is an economically-important legume (Fabaceae). The example shown here is of amature stem at the end of primary growth, that is, the vascular bundles contain very limited amounts of secondary xylem and secondary phloem. The cortex is very narrow and is composed of chlorenchyma. The cortex is separated from the vascular bundles and the underlying pith, by a starch sheath. The pith is parenchymatous.
Fig. - 2show a detail of part of the stem, and two adjacent vascular bundles.The epidermis (EP) is thin-walled and beneath this are four-five rows of chlorenchyma. The cortexes is separated from the stele by a starch sheath (SS) and immediately beneath this, are very conspicuous phloem fiber caps (PF), which overlie a narrow band of predominantly primary phloem (P). A fascicular cambium zone (CZ) has produced a few secondary xylem elements, but apparently, no secondary phloem (inset, below right). The stele (St) is entirely parenchymatous
(virtualplant.ru.ac.za/Main/ANATOMY/trifolium_stem.htm)
TRADITIONAL USE
Red clover has been used traditionally as a medicinal agent by Asian, European, and Native American cultures as an expectorant in asthma, and as an alternative (blood purifier) to treat psoriasis, eczema, and other chronic skin conditions.
MEDICINAL USES AND INDICATIONS
Red clover is a source of many nutrients including calcium, chromium, magnesium, niacin, phosphorus, potassium, thiamine, and vitamin C. Red clover is a rich source of isoflavones (chemicals that act like estrogens and are found in many plants).
THERAPEUTIC USES
- Red clover has a long history of traditional use in China, Europe and Russia for a variety of medical conditions including bronchial asthma, liver and digestive ailments, sore eyes and burns. It was thought to purify the blood by promoting urine, mucus and bile production and improve circulation.
- Traditionally red clover ointments have been applied to the skin to treat conditions such as psoriasis and eczema.
- There is a history of use of red clover as a short-term cough medicine for children. Red clover is a rich source of the isoflavones; formonnectin, biochanin A, daidzin and genistein which are natural hormones.
- Red clover supplements are promoted worldwide for the treatment of menopausal symptoms and the maintenance of health and welfare after the menopause.
- Clinical trials show that red clover extracts may provides some benefit for hot flushes associated with the menopause.
- It is also suggested that Red Clover may be of use for women experiencing breast pain.
- There is promising evidence for a positive role for red clover in the development of osteoporosis.
- Red clover extracts have been shown to have a positive effect on high blood pressure.
- Clinical studies have shown red clover to have no detrimental effect on the womb or breast tissue.
- In addition it’s natural hormonal components; Red clover contains many essential vitamins and minerals like calcium, chromium, niacin, phosphorous, potassium, thiamine, magnesium, and vitamin C.
- Red clover can be found in herbal combinations thought to be useful for women’s health.
- (streetdirectory.com/food_editorials/cooking/herbs_and_spices/red_clover_trifolium_pratense_side_effects_and_benefits.html)
CHEMICAL CONSTITUENTS
35.54% isoflavones, 1.11% flavonoids, 0.06% pterocarpans, Less than or equal to 0.03% coumarins, tyramine, Daidzein, Genistein, formononetin, biochanin A, coumestrol, naringenin . (Karjalainen et. al, August 2009)
Structures of main phenolics found in red clover leaves
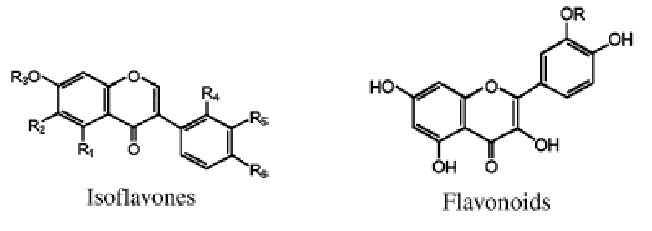
|
Isoflavones |
R1 |
R2 |
R3 |
R4 |
R5 |
R6 |
|
Afrormosin |
H |
OCH3 |
H |
H |
H |
OCH3 |
|
Biochanin A |
OH |
H |
H |
H |
H |
OCH3 |
|
Calycosin |
H |
H |
H |
H |
OH |
OCH3 |
|
Daidzein |
H |
H |
H |
H |
H |
OH |
|
Formononetin |
H |
H |
H |
H |
H |
OCH3 |
|
Genistein |
OH |
H |
H |
H |
H |
OH |
|
Irilin B |
OH |
OCH3 |
H |
OH |
H |
H |
|
Irilone |
OH |
–O– |
- CH2– |
H |
H |
H |
|
Methylorobol |
OH |
H |
H |
H |
OCH3 |
OH |
|
Pratensein |
OH |
H |
OH |
H |
OH |
OCH3 |
|
Prunetin |
OH |
H |
CH3 |
H |
H |
OH |
|
Pseudobaptigenin |
H |
H |
H |
H |
–O– |
–OCH2– |
|
Texasin |
H |
OH |
H |
H |
H |
OCH |
|
Flavonoids |
R |
|
|
|
|
|
|
Kaempferol |
H |
|
|
|
|
|
|
Quercetin |
OH |
|
|
|
|
|
(Karjalainen et. al, August 2009)
SPECTROSCOPIC DATA BY USING HPLC-MS:
|
S. No. |
tR(min) |
λmax(nm) |
[M+H]+ |
[M+Na]+ |
[2M+Na]+ |
Tentative identification |
|
1. |
12.4 |
300 ,325 |
|
319 |
615 |
Phaselic acid (cis/trans) |
|
2. |
12.33 |
300, 325 |
|
319 |
615 |
Phaselic acid (cis/trans) |
|
3. |
16.58 |
289 ,320 |
360 |
382 |
|
cis-Clovamide |
|
4. |
17.44 |
250, 300 |
417 |
439 |
|
Daidzein-G |
|
5. |
19.48 |
250, 260, 287 |
447 |
469 |
|
Calycosin-G |
|
6. |
21.52 |
260, 320 |
433 |
455 |
|
Genistein-G |
|
7. |
22.32 |
262, 334 |
463 |
485 |
|
Pratensein/Irilin B/Methylorobol-G |
|
8. |
22.92 |
260, 330 |
519 |
541 |
|
Genistein-G-M |
|
9. |
23.53 |
250, 300 |
503 |
525 |
1027 |
Daidzein-G-M |
|
10. |
24.37 |
255, 353 |
465 |
487 |
|
Quercetin-galactoside |
|
11. |
24.72 |
255, 353 |
465 |
487 |
|
Quercetin- glucoside |
|
12. |
25.04 |
250, 287 |
533 |
555 |
|
Calycosin-G-M |
|
13. |
25.29 |
250, 295 |
431 |
453 |
883 |
Formononetin-G |
|
14. |
25.71 |
255, 353 |
551 |
573 |
|
Quercetin-G-M |
|
15. |
26.14 |
250,300 |
517 |
539 |
1055 |
Formononetin-G-M |
|
16. |
26,71 |
265, 350 |
449 |
471 |
|
Kaempferol-G |
|
17. |
27.31 |
330 |
519 |
541 |
1059 |
Genistein-G-M |
|
18. |
27.78 |
263,340 |
549 |
571 |
|
Pratensein/Irilin B/Methylorobol-G-M |
|
19. |
28.98 |
260, 285, 330 |
549 |
571 |
|
Pratensein/Irilin B/Methylorobol-G-M |
|
20. |
30.16 |
250, 260, 280 |
531 |
553 |
1083 |
Pseudobaptigenin-G-M |
|
21. |
30.92 |
250, 300 |
517 |
539 |
1055 |
Formononetin-G-M |
|
22. |
31.45 |
285, 310 |
533 |
555 |
|
Maackiain-G-M |
|
23. |
31.71 |
260,330 |
533 |
555 |
1087 |
Prunetin/Texasin-G-M |
|
24. |
32.58 |
270, 340 |
547 |
569 |
|
Irilone/Afrormosin-G-M |
|
25. |
33.34 |
260, 330 |
271 |
293 |
|
Genisteina |
|
26. |
35.00 |
262 |
301 |
323 |
|
Pratensein/Irilin B/Methylorobol |
|
27. |
36.10 |
285, 310 |
285 |
301 |
|
Maackiain |
|
28. |
36.58 |
260, 325 |
533 |
555 |
1087 |
Biochanin A-G-M |
|
29. |
37.58 |
250,302 |
269 |
291 |
559 |
Formononetin |
|
30. |
42.45 |
260, 325 |
285 |
307 |
|
Prunetin/Texasina |
|
31. |
43.84 |
260, 325 |
285 |
307 |
|
Biochanin A |
(Karjalainen et. al, August 2009)
REPORTED PHARMACOLOGICAL ACTIVITY
1. Biological estimation of oestrogenic activity in red clover (Trifolium pratense): relative potencies of parts of plant and changes with storage
Immature ovariectomized mice were used to compare the oestrogenic activities of different parts of the same clover plants; to estimate the relative potencies of biochanin A, genistein and diethylstilboestrol; and to compare the effects of different methods of storage on the oestrogenic potency of red clover leaf and petiole. Test materials were incorporated in the diet fed to the mice and the uterine weight response was used to measure oestrogenic activity.
Conclusion: Comparison of successive estimates of the relative potency of isoflavones and diethylstilboestrol indicated that the relative responsiveness to the two types of oestrogen did not remain constant. Thus comparisons of estimates of oestrogenic activity of plant material, obtained in terms of diethylstilboestrol in different experiments, could be invalid.
(D. S. Flux et.al, June, 2009)
2. An ovariectomized rat model to determine the estrogenic and antiestrogenic activity of Trifolium pratense L. (red clover) extracts
A red clover extract, standardized to contain 15% isoflavones was administered by gavage [250, 500 and 750 mg/(kg x d)] to virgin, ovariectomized 50-d-old Sprague-Dawley rats, for 21 d in the presence and absence of 17beta-estradiol [50 micro g/(kg x d)]. Estrogenic effects included an increase in uterine weight, vaginal cell cornification and mammary gland duct branching. Red clover produced a dose-dependent increase in uterine weight and differentiated vaginal cells at the two higher doses, but it did not stimulate cell proliferation in the mammary glands. Neither antiestrogenic nor additive estrogenic properties were observed in any of the tissues studied.
Conclusion: These data suggest that red clover extract is weakly estrogenic in the ovariectomized rat model and red clover didn’t produce any additive estrogenic or antiestrogenic activity when administered together with 17β-estradiol. (Burdette et.al, January, 2002)
3. Antioxidant Profile of Trifolium pratense:
Examination of the antioxidant properties of five different extracts of Trifolium pratense leaves, various assays which measure free radical scavenging ability were carried out: 1,1-diphenyl-2-picrylhydrazyl, hydroxyl, superoxide anion and nitric oxide radical scavenger capacity tests and lipid peroxidation assay . In addition, in vivo experiments were conducted with antioxidant systems (activities of GSHPx, GSHR, Px, CAT, XOD, GSH content and intensity of LPx) in liver homogenate and blood ofmice after their treatment with extracts of T. pratense leaves, or in combination with CCl4. Besides, in the extracts examined the total phenolic and flavonoid amounts were also determined, together with presence of the selected flavonoids: quercetin, luteolin, apigenin, naringenin and kaempferol, which were studied using a HPLC-DAD technique.
Conclusion:
Antioxidant activity results show that the EtOAc and H2O extracts of T. pratense leaves are efficient in protection of tissues and cells from oxidative stress. Based on these results it can be concluded that the H2O and EtOAc extracts of leaves of T. pratense showed strong antioxidant activities when compared to the standards. However, the difference in the antioxidant activities of these two extracts may be due to their different phytochemical composition. The results obtained emphasize that the H2O and EtOAc extracts mainly exhibit their antioxidant potential via free radical scavenging and electron donation. .The T. pratense leaves extracts exhibited differentactivities in relation to the investigated biochemical parameters. The results obtained indicate toxicityof CCl4, probably due to the radicals involved in its metabolism. In in vivo experiments the best protective effect was shown by the EtOAc and H2O extracts. Therefore, it seems reasonable to consider these leaf extracts as a new valuable source for pharmaceuticals in the promotion of health as commercial drugs.(Kaurinovic et.al, September,2012)
4. Trifolium pratense isoflavone biochanin A inhibits aromatase activity and expression:
The effect of biochanin A on the gene regulation and enzyme activity of aromatase was investigated. By assaying MCF-7 cells stably transfected with CYP19, biochanin A inhibited aromatase activity and hampered cell growth attributing to the enzyme activity. In addition, 25 microm-biochanin A significantly reduced CYP19 mRNA abundance in the oestrogen receptor-negative breast cancer cells SK-BR-3. The transcriptional control of the CYP19 gene is exon-specific, and promoter regions I.3 and II have been shown to be responsible for CYP19 expression in SK-BR-3 cells. Luciferase reporter gene assays also revealed that biochanin A could repress the transcriptional control dictated by the promoter regulation. Interestingly, genistein did not inhibit aromatase but it might down regulate promoter I.3 and II transactivation. biochanin A, it might contribute to biochanin A's suppressive effect on CYP19 expression.
Conclusion: biochanin A inhibited CYP19 activity and gene expression. (Wang et.al.,Aug, 2007).
5. (Trifolium pratense) phase II clinical extract possesses opiate activity:
Trifolium pratense (TP) is one of the most common herbs for the relief of menopausal symptoms. In this study, investigated the affinity of TP at the mu- and delta-opiate receptors. TP extract bound to the mu-opiate receptor with a high affinity (K (i) =9.7+/-1.6microg/ml). The same extract was also found to have affinity at the delta-opiate receptor with K (i) of 15.9+/-2.4microg/ml.
Conclusion: These results for the first time suggest a potential new mechanism of action of TP at the opiate receptors. Th.e essential role of the opioid system in regulating temperature, mood, and hormonal levels and actions, this may explain in part the beneficial effect of TP in alleviating menopausal symptoms.(Nissan et.al, June ,2007)
6. Determination in vivo and in vitro anti-inflammatory activity of red clover dry extract:
The in vitro anti-inflammatory activity was assayed by the technique using the Boyden chamber method, evaluating the leukocyte migration inhibition (chemotaxis). The in vivo anti-inflammatory activity was tested by a carrageenan-induced rat paw edema test.
Conclusion: The anti-inflammatory in vitro test showed that there was a significant inhibition ofleukocyte migration at the concentrations of 100, 50, 25, 10 and 5 μg/mL of redclover dry extract, these doses resulted in 94.73, 95.39, 94.73, 84.68 and 78.75% ofinhibition for each dose, respectively. The anti-inflammatory in vivo test resultedin a significant activity in both tested doses (100 and 50 mg/kg of red clover dryextract) and at each tested time. The average percentage of edema inhibition was63.37%. The findings of this study suggested that red clover extract might be suitable for the treatment of inflammatory diseases.
(Graziele P. Ramos, et.al, Nov, 2011)
ACKNOWLEDGEMENT:
I would like to record my gratitude to my esteemed respected guide Dr. (Prof.) Shalini Tripathi, Department of Pharmacy, Rameshwaram Institute of Technology and Management
REFERENCE
• Burdette,J.E,., Jianghua L., Lantvit ,D., Eula, L., Nancy, B., Krishna, P., Bhat,L., Hedayat,S., Richard, B., Breemen,V., Andreas, I., John, M., Norman R., Farnsworth & Bolton, J.L,2002.Publication: J. Nutr. 132 pp::27-30.
• Carlsson,G., Palmborg,C., Jumpponen,A., Högberg, P & Huss-Danell, K. 2005. N2 fixation in three perennial Trifolium species in communities of varied plant species richness.Input from N2fixation to northern grasslands. Acta Universitatis agriculturae Sueciae ,pp: 2005:76.
• Duke, J.A., Ayensu, E.S., 1985. Medicinal Plants of China.
• Flux,D. S.,Munford, R.E.,Wilson,G.F,1962. Massey University College of Manawatu, Palmerston North, New Zealand.
• Graziele,P., Ramos,M. A., Apel,C.B.,Morais,P.C.Ceolato,E.E.,Schapoval S.,,Miguel D.A., Zuanazzi,A.S., 2012. Revista Brasileira de Farmacognosia Brazilian Journal of Pharmacognosy.pp: 176-180.
• Hagsand, E.,Wik, E. 1968.Variety trials with as like clover and red clover in central and northern Norrland,pp: 1-72.
• Hamrick,JL., Linhan,YB.,Mitton.,JB,1979.Relationships between life history Characteristics and electrophoreticaly deteetable genetic variation in plants pp:173-200.
• (plants.usda.gov/java/profile?symbol=trpr2)
• (akuna.net/picture/Canada_WEB/PDFs/RED%20CLOVER.pdf)
• (virtualplant.ru.ac.za/Main/ANATOMY/trifolium_stem.htm)
• Karjalainen,R.O., Oksanen,E., Tiitto,R.J., Savirant., Niina,M.M..,2009. Journal of the Science of food and Agriculture, Vol 90,issue 3. pp: 418-423.
• Kaurinovic, B.,Popovic,M., Vlaisavljevic, S., Schwartsova , H.,2 and Vojinovic, M.,- Milorado 2012. ISSN 1420-3049 .
• LaRue,TA., Patterson,TG.,1981. How much nitrogen do legumes flX. pp: 15-38.
• Nissan,H.P.,JianL., Booth,N.L.,Yamamura,H.I.,Farnsworth,N.R.,Wang,Z.J,2007.Department of Biopharmaceutical Sciences, University of Illinois at Chicago,USA..
• Nordstedt, O.1920. Prima loca plantarum suecicarum. Första littera turupp gift om de i Sverige funna vilda eller förvildade kärlväxterna,pp: 1-95.
• Norman, R., Farnsworth b, Jim, Z., Wang,a. 2007.Department of Biopharmaceutical Sciences, University of Illinois at Chicago,USA. Journal of Ethnopharmacology .pp:112.
• Piersen,C.E.,Booth,N.L.,Sun,Y.,Liang,W.,Burdette,J.E.,VanBreemen,R.B.,Geller,S.E.,Gu,C.,Banuvar, S., Shulman, L.P., Bolton, J.L., Farnsworth, 211 N.R., 2004. Chemical and biological characterization and clinical evaluation 212 of botanical dietary supplements: a phase I red c lover extract as a model. 213 Current Medicinal Chemistry 11, pp: 1361–1374.
• Smith,RR.,Taylor,NL.,Bowley,SR.,1985,Red clover. ln: NL Taylor(ed), Clover science and technology, pp: 457-469.
• Taylor, NI.,Smith,RR.,1979.Red clover breeding and genetics, pp: 125-153.
• Taylor, N.L.,Quesenberry, K.H., 1996. Red clover science. Current plant science and biotechnology in agriculture 28. Kluwer Academic Publishers, Dordrecht, Netherlands. pp:226 .
• Wang,Y., Man,GW., Chan, FL., Chen, S., Leung, LK, 2008 . Department of Biochemistry, The Chinese University of Hong Kong, Shatin NT, Hong Kong.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 3 Received On: 22/01/2014; Accepted On: 30/01/2014; Published On: 05/03/2014 How to cite this article: N Chaudhary, S Tripathi, A review on chemical and biological activity of Trifolium Pretense, PharmaTutor, 2014, 2(3), 93-101 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









