About Author:
Sarabjeet Kaur
M.pharm Lord Shiva College of pharmacy Sirsa
Teaching Assistant Dept of Pharm. Edu. and research, Bhainswal Kalan
BPSMV, Sonipat
jassarab@gmail.com
ABSTRACT:
Asymmetric hydrogenation is by far the most attractive form of stereoselective synthesis. Using optically pure catalysts, prochiral substrates can be converted to chiral compounds with high selectivity. These catalysts can be completely organic in nature (for example proline 50) or contain a transition metal which coordinates to an organic chiral ligand. In the last decades, considerable progress has been made in the development of metal catalyzed asymmetric transformation based on enantiopure ligands complexed to a transition metal core. However, the identification of suitable asymmetric catalysts still poses one of the most challenging endeavours in contemptory chemistry.
REFERENCE ID: PHARMATUTOR-ART-1642
INTRODUCTION:
Asymmetric hydrogenation potentially represents one of the most powerful and cost effective method for producing enantiomerically enriched compound. A great number of optically active compounds contain a hydrogen atom at the stereogenic centre and this hydrogen atom can be introduced into appropriate unsaturated precursor by hydrogenation. It involves the application of asymmetric catalysts. Asymmeteric catalyst compose of transition metal bound to chiral ligand. General mechanism of transition metal complex in asymmetric hydrogenation is explained in Figure 1.
Under reaction conditions, the initially used precatalyst 1 is converted to the true catalyst 2 (induction process) that activates achiral molecules A and B and transforms them to the chiral product A–B (catalytic cycle). Some of the steps in the multistep transformation are reversible, whereas the first is irreversible step, and the step 3→4, determines the absolute stereochemistry of A–B product.
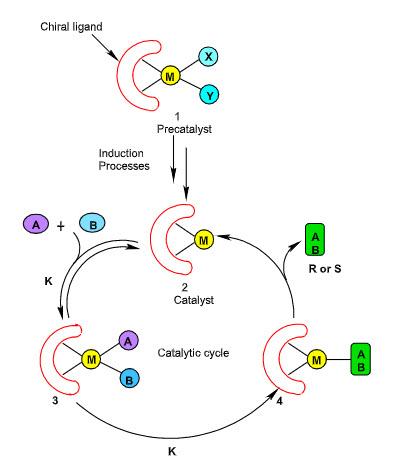
Figure 1
Efficient asymmetric catalysis requires efficient selection of a chiral ligand and transition metal complex because they can effect a desired transformation with both high efficiency and high selectivity. Selection of asymmetric catalyst can be divided into two parts:
Selection of transition metal catalyst: Most of the elements that have proved valuable in forming compounds suitable for catalytic homogeneous reductions form part of the second transition series in the periodic table. Complexes of Pd, Pt, Ru, Ir, Rh, Fe, Ni, and Co can be used as catalysts forasymmetric hydrogenation. Generally, the most active catalysts for reduction are Rh, Ru, and Pd but the preffered one is rhodium. Selection of chiral ligand: Selection of an excellent chiral ligand is also crucial for high-performance asymmetric catalysis. There are number of chiral ligands with different backbones which had been successfully applied in the past for asymmetric hydrogenation such as DIOP; DIPAMP;Chiraphos; BINAP; DuPhos and BPE (Figure 2).
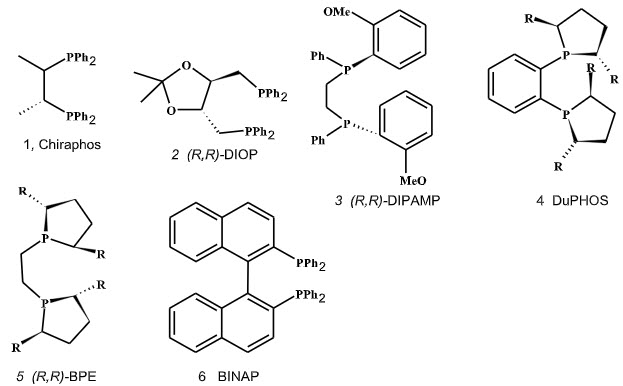
Figure 2
A great achievement in the asymmetric hydrogenation was achieved with the advent of BINAP.It has been reported in the literature that BINAP serves as excellent chiral ligand for asymmetric hydrogenation of various olefinic substrate. BINAP is a fully aromatic, axially dissymmeteric C2 chiral diphosphine that would exert strong steric and electronic influences on transition metal complexes. This ligand was first used in rhodium-catalyzed asymmetric hydrogenation of α-(acylamino)acrylic acids, in which high selectivities were reported for certain substrates.
Transition metal complexes containing BINAP as atropoisomeric C2 chiral diphosphine, where rotation around the single bond is restricted because of steric factor, exhibit exceptionally high chiral recognition in various catalytic reactions, opening tremendous potential for stereoselective organic synthesis.
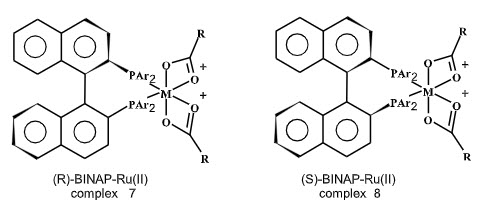
Figure 3
Various reports have been published in which this approach has been exploited. Initial reports regarding the success of asymmetric hydrogenation was reported by Knowles et al. in 1970 who had developed the first industrial process for the synthesis of
L-DOPA (which is widely used in the treatment of Parkinson’s disease). The ligand used in industrial synthesis of L-DOPA in the early 1970s was the diphosphine ligand DIPAMP. A rhodium complex with the ligand DIPAMP gave a mixture of the enantiomers of DOPA in 100% yield (Scheme 1). The product contained 97.5% of L-DOPA.
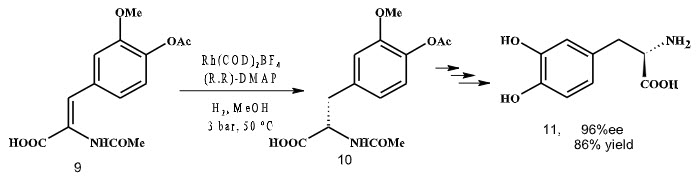
Scheme 1
The next real breakthrough in area of asymmetric hydrogenation was given by Noyori and co-workers in 1986. He had demonstrated that the well-defined, mononuclear Ru-BINAP catalysts had an incredible range of efficiency for asymmetric hydrogenation of various olefinic and other substrate. They had reported the asymmetric hydrogenation of α-(acylamino) acrylic acid catalyzed by Ru dicarboxylate complex with moderate enantioselectivity (Scheme 2).
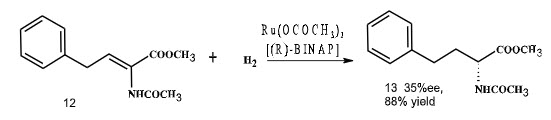
Scheme 2
In 1987, Zhang et al have developed a novel class of bisphosphinite ligands like (o-BINAPO) (Figure 4) for enantioselective hydrogenation of β-aryl-substituted 3-aminoacrylates.
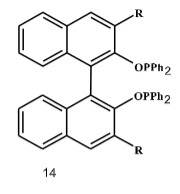
Figure 4
In 1988, Zhang et al. have developed a straightforward process to prepare β-amino esters via rhodium catalyzed hydrogenation of β-aminoacrylates (Scheme 3).
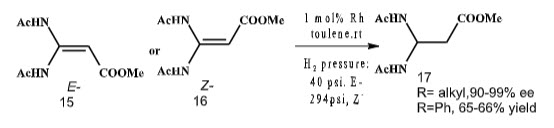
Scheme 3
In 1989, Noyari et al. had developed a process for the production of acetoxyazetidinone (a key intermediate in the synthesis of antibiotic) by combining asymmetric hydrogenation with dynamic kinetic resolution. (Scheme 4).
Scheme 4
In 1991, Blaser et al. had optimized reaction conditions for the hydrogenation of ethyl 2-oxo-4-phenyl-butyrate, intermediate for the ACE inhibitor Benazepril and the best optical yields obtained were 96%. Ligand used was Norphos with rhodium as a catalyst (Scheme 5).
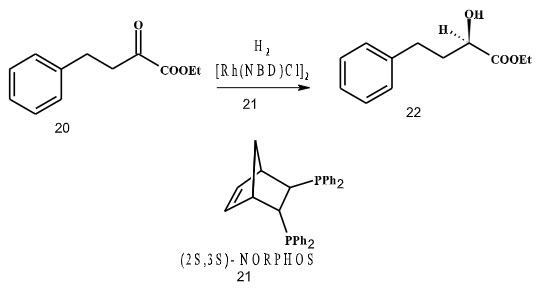
Scheme 5
In 2001, Gridnev and Imamoto had reported the achievement of excellent (R)-enantioselectivity by using electron rich P-chirogenic diphosphines-Rh complexes in the asymmetric hydrogenation of (E)-β- alkyl β-aminoacrylates (Scheme 6).
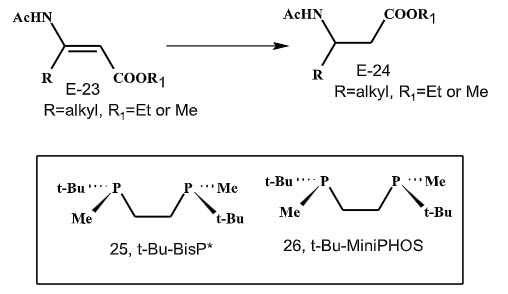
Scheme 6
In 2001, Jackson and coworkers had reported an enantioselective hydrogenation of α,β-unsaturated nitriles and their methyl esters bearing an α-phthalimidomethyl substituent (Scheme 7).
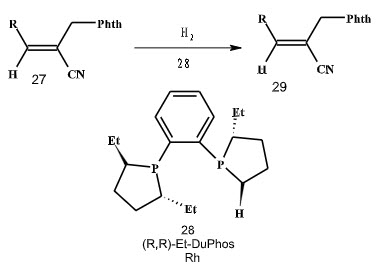
Scheme 7
In 2002, Feringa and coworkers described the use of monodentate phosphoramidite ligands in the rhodium-catalyzed hydrogenation of (E)/(Z)-β-dehydroamino acids (Scheme 8). Ligand 32 leads to β-amino acid precursors with excellent enantioselectivities for (E)-substrates, while a slight modification in the amine backbone of the ligand i.e. 33 leads to very good enantioselectivities in the hydrogenation of (Z)-substrates.
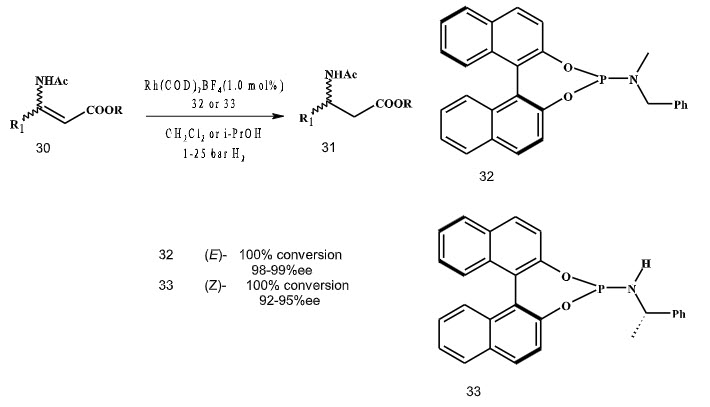
Scheme 8
In 2003, Borner and coworkers used 1,3-diphenyl 1,3 bis (diphenylphosphino) propane in the hydrogenation of (E)-enamines to give β-amino esters with up to 97% ee. However(Z)-Enamines as substrates lead to significasntly lower enantioselectivities (Scheme 9).
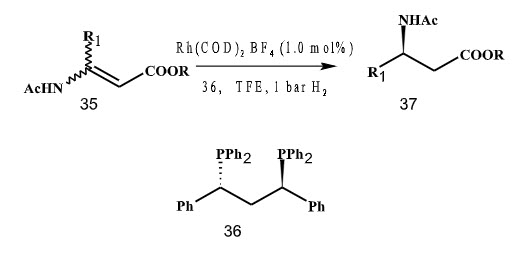
Scheme 9
Ikemoto et al. (2004) had reported the diastereoselective heterogeneous catalytic system that converts chiral phenylglycinamide (PGA) to β-amino acid derivative in upto 99% d.e (Scheme 10).
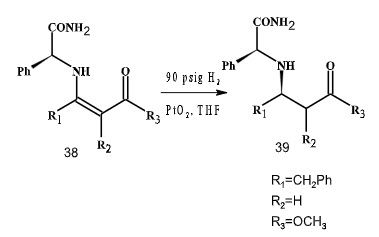
Scheme 10
A practical hydrogen pressure of 90 psi was used at 22oC. The reaction with 10 mol % PtO2 proceeded slow. Acetic acid was used to accelerate hydrogenations catalyzed by PtO2 by activating the catalyst. Addition of increasing amounts of acetic acid lead to improved rates but resulted in a progressive decline in selectivity.
In 2004, Hsio and co-workers have given an important contribution in the area of asymmetric hydrogenation. They utilized chiral ferrocenyl phosphine ligand in the hydrogenation of (Z)-enamine esters with an unprotected amine group in trifluoroethanol (TFE) as solvent to yield the corresponding amino esters with excellent ee (up to 97%) (Scheme 11). This represents the first example of a high yielding enantioselective hydrogenation of unprotected enamine esters without the use of a directing protecting group.
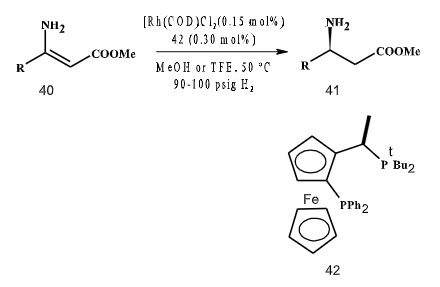
Scheme 11.
In 2005, Zhang and co-workers have reported asymmetric hydrogenation of both
α-aryl and α-alkyl-α-(acylamino) acrylates with excellent enantioselectivities and high turnovers with a new unsymmetrical hybrid Tang phos ligand with rhodium catalyst (Scheme 12).
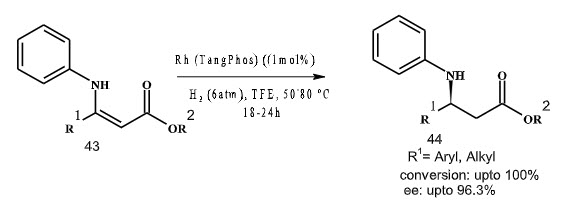
Scheme 12
Shultz et al. (2009) have reported a process for asymmetric hydrogenation of β-dehydroamino acid by using rhodium catalyst with Josiphos as ligand, with 98% ee, although with low to medium TONs and TOFs (Scheme 13).
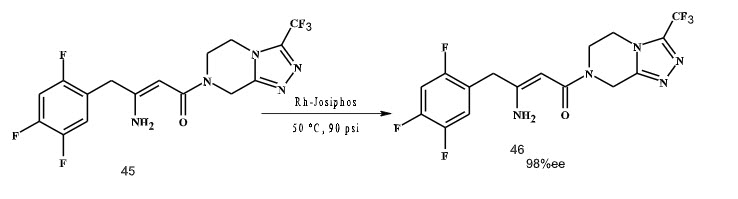
Scheme 13
Conclusion
In the past 15 years, tremendous progress has been made as shown in this review and preceeding overviews. Organocatalysis has played an important role in recent years in the field of catalytic asymmetric synthesis and quickly provided a useful method to prepare enantiomerically pure compound from achiral compound. However, in many cases high catalyst loadings have to be used, and reaction times are in some cases rather long. On the other hand, one of the more promising catalysts, (S)-proline, is a naturally occuring amino acid, and therefore very cheap. Homogeneous catalysis using transition metals provided the most important methods in recent years for synthesis. Among them, the hydrogenation of enamines has been applied in industrial synthesis of β-amino acids because high turnover, high enantioselectivity and low catalyst loadings can be used. However, some transition metals are highly toxic and harmful for the environment. Therefore, biocatalysis would provide an important alternative, but up to now no enzymes areknown that have a broad substrate scope in combinations with high turnover numbers. Most enzymatic methods rely on kinetic resolutions, which means that only 50% of the starting materials can be converted, unless a dynamic kinetic resolution protocol is used. Therefore, the catalytic asymmetric synthesis of enantiomerically pure compound starting from simple and cheap starting materials and using recycable sustainable catalysts remains an important challenge for synthetic organic chemists.
References
1. Genet J P. Asymmetric catalytic hydrogenation. Design of new Ru catalysts and chiral ligands: From laboratory to industrial applications Acc. Chem Res. 2003; 36: 908-918.
2. Noyori R, Kitamura M, Ohkuma T. Toward efficient asymmetric hydrogenation:Architectural and functional engineering of chiral molecular catalysts. PNAS. 2004; 101: 5357.
3. Genet J P. Recent studies on asymmetric hydrogenation. New catalysts and synthetic applications inorganic synthesis. Chem. Pure Appl. 2002; 74: No.77–83.
4. Knowles W S, Noyori R. Pioneering perspectives on assymmetric hydrogenation. Acc Chem Res 2007; 40: 1238-1239.
5. Berthod M, Mignani G, Woodward G, Marc Lemaire M. Modified BINAP: The how and the why. Chem Rev. 2005; 105: 1801-1836.
6. D. Blanc D, Henry J C, Vidal V R, Genet J P. Skewphos-Ru(II): An efficient catalyst for asymmetric hydrogenation of functionalized ketones. Tetrahedron Lett. 1997; 38: 6603.
7. Vineyard B D, Knowles W S, Sabacky G L. Asymmetric hydrogenation using rhodium chiral biphosphine catalyst. J. Am. Chem. Soc. 1977; 99: 5946-5947.
8. Miyashita A, Yasuda A, Takaya H, Toriumi K, Ito T, Souchi T, Noyori R. Synthesis of 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of .alpha.-(acylamino)acrylic acids. J Am Chem Soc. 1980; 102: 7932.
9. Zhu G, Chen Z, Zhang X. Highly efficient asymmetric synthesis of β-amino acid derivatives via rhodium catalyzed hydrogenation of β-(Acylamino) acrylates. J Org Chem. 1999; 64: 6907–6910.
10. Noyori R, Ohta M, Hsiao Y, Kitamura M, Ohta T, Takaya H. Stereoselective hydrogenation via dynamic kinetic resolution. J Am Chem Soc. 1986; 108: 7117.
11. Spindler F, Blaser H U. Enantioselective reduction of C=N bonds and enamines with hydrogen, in transition metals for organic synthesis. J. Wiley & Sons. 1998; 2: 69-78.
12. Yasutake M, Gridnev I D, Higashi N, Imamoto T. Highly enantioselective hydrogenation of (E)-β-(Acylamino) acrylates catalyzed by Rh (I)-complexes of electron-rich P-chirogenic diphosphines. Org Lett. 2001; 3: 1701–1704.
13. Saylik D, Campi E M, Donohue A C, Jackson W R, Robinson A J. Highly diastereoselective synthetic route to enantiopure β-amino acids and γ-amino alcohols using a fluorinated oxazolidine (Fox) as chiral auxiliary. Tetrahedron: Asym. 2001; 12: 657–667.
14. Adriaan J M, Feringa B L. Asymmetric hydrogenation using monodenatate phosphoamidite ligand. J Am Chem Soc. 2007; 40(12): 1267-1277.
15. Ikemoto N, Tellers D M, Dreher S D, Liu J, Haung A, Rivera N R, Njolito E, Hsiao Y, McWilliams J C, Williams J M, Armstrong J D, Sun Y, Mathre D J, Grabowski E J J, Tillyer R D. Highly distereoselective heterogenously catalyzed hydrogenation of enamines for the synthesis of chiral β-amino acid derivatives. J Am Chem Soc. 2004; 126: 3048-3049.
16. Hsiao Y, Rivera N R, Rosner T, Krska S W, Njolito E, Wang F, Sun Y, Armstrong J D, Grabowski E J J, Tillyer E D, Spindler F, Malan C. Highly efficient synthesis of β-amino acid derivatives via asymmetric hydrogenation of unprotected enamines. J Am Chem Soc. 2004; 126 (32): 9918–9919.
17. Hu X P, Zheng Z. Practical Rh(I)-catalyzed asymmetric a hydrogenation of β-(Acylamino) acrylates using a new unsymmetrical hybrid ferrocenylphosphine-phosphoramidite ligand: Crucial influence of an N-H proton in the ligand. Org Lett. 2005; 7: 419-422.
18. Shultz C S, Krska S W. Unlocking the potential of asymmetric hydrogenation at Merck. Acc Chem Res. 2007; 40: 1320-1326.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









