 About Authors:
About Authors:
H. S. Sawwalakhe*, J. M. Maidankar, M. A. Channawar, Dr. A. V. Chandewar
P. W. College of Pharmacy, Yavatmal,
Amravati university
*hemant_11sep@rediffmail.com
ABSTRACT:
Occular drug delivery is one of the most interesting and challenging endeavors facing the pharmaceutical scientists, the major problem encountered to pharmaceutical scientist is rapid precorneal elimination of the drug, resulting in poor bioavailability and therapeutic response, because of high tear fluid turnover and dynamics. In situ-forming gels are liquid upon instillation and undergo phase transition in the ocular cul de-sac to form visco-elastic gel and these gels provides a response to environmental changes. In the past few years, an impressive number of novel temperature, pH, and ion induced in situ-forming systems have been reported for sustained ophthalmic drug delivery. Each system has its own advantages and drawbacks. The choice of a particular gel depends on its intrinsic properties and envisaged therapeutic use. This review includes various temperature, pH, and ion induced in situ-forming polymeric systems used to achieve prolonged contact time of drugs with the cornea and increase their bioavailability Now a days in situ gel have been used as vehicles for the delivery of drugs for both local treatment and systemic effects. Different administration routes other than ocular have been explored, and these cutaneous and subcutaneous delivery, dental, buccal delivery and delivery to the esophagus, stomach, colon, rectum and vagina.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1313
INTRODUCTION:
The main aim of pharmacotherapeutics is the attainment of effective drug concentration at the intended site of action for a sufficient period of time to elicit the response. A major problem being faced in ocular therapeutics is the attainment of optimal concentration the site of action. Poor bioavailability of drugs from ocular dosage forms is mainly due to the tear production, transient residence time, and impermeability of corneal epithelium.1
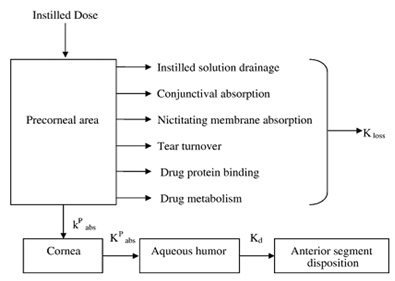
Figure 1: Model depicting precorneal and intraocular drug movement from topical dosing.
The poor bioavailability and therapeutic response exhibited by conventional ophthalmic solutions due to rapid precorneal elimination of the drug may be overcome by the use of a gel system that are instilled as drops into the eye and undergo a sol-gel transition from the instilled dose. 2
The following characteristics are required to optimize ocular drug delivery systems.3
* A good corneal penetration.
* A prolonged contact time with corneal tissue.
* Simplicity of installation for the patient.
* A non-irritative and comfortable form (the viscous solution should not provoke lachrymation and reflex blinking)
Initial attempts to overcome the poor bioavailability of topically instilled drugs typically involved the use of ointments based on mixtures of white petrolatum and mineral oils and suspensions.4, 5 Ointments ensure superior drug bioavailability by increasing the contact time with the eye, minimizing the dilution by tears, and resisting nasolachrymal drainage because these vehicles have the major disadvantage of providing blurred vision, they are nowadays mainly used for either night time administration or for treatment on the outside and edges of the eyelids.6 Use of suspensions as ophthalmic delivery systems relies on the assumption that particles may persist in the conjunctival sac. The efficiency of suspensions has shown high variability, which occurred as a result of inadequate dosing, probably mainly due to the lack of patients compliance in adequately shaking the suspension before administration.7
These disadvantages have led to other approaches being investigated. One of the common methods to optimize prolonged precorneal residence time is to use hydrogels, liposomes, micro and nano carrier systems. In comparison with traditional formulations, these systems have the advantages of 8, 9 10.11
* Prolonged drug release
* Reduced systemic side effects
* Reduced number of applications
* Better patient compliance.
Even though various drug delivery systems mentioned above offer a numerous advantages over conventional drug therapy but still they are not devoid of pitfalls, including
* Poor patient compliance and difficulty of insertion as in ocular inserts.
* Tissue irritation and damage caused by penetration enhancers and collagen shields.
* Toxicity caused by insertion of foreign substances like albumin and poly butyl cyano acrylate, as in case of nano particles and microspheres.
* Change in pharmacokinetic and pharmacodynamics of the drug as caused by altering the chemical structure of the drug (prodrug approach)
[adsense:468x15:2204050025]
HYDROGEL:
The most common way to improve drug retention on the corneal surface is undoubtedly by using polymers to increase solution viscosity. Hydrogels are polymers endowed with an ability to swell in water or aqueous solvents and induce a liquid–gel transition. Currently, two groups of hydrogels are distinguished, namely preformed and in situ forming gels. Preformed hydrogels can be defined as simple viscous solutions which do not undergo any modifications after administration. In situ forming gels are formulations, applied as solutions, sols, or Suspensions, that undergo gelation after instillation due to physicochemical, changes inherent to the eye. The objective of this review is to describe the various temperature, pH, and ion induced, in situ-forming polymeric systems used to achieve prolonged contact time of drugs with the cornea and increase their bioavailability.13, 14
Currently two groups of hydrogels are distinguished (Figure 2), namely preformed and in situ forming gels. Preformed hydrogels can be defined as simple viscous solutions, which do not undergo any modifications after administration, while in situ forming gels are formulations, applied as a solution, which undergoes gelation after instillation due to physico-chemical changes inherent to the eye.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
In situ gel:
The use of preformed hydrogels still has drawbacks that can limit their interest for ophthalmic drug delivery. They do not allow accurate and reproducible administration of quantities of drug. They often produce blurred vision, crusting of eyelids and lachrymation upon administration.
Distinguishing from preformed hydrogels, in situ forming gels are formulations, applied as a solution, which undergoes gelation after instillation due to physicochemical changes inherent to the biological fluids. In this way, the polymers, which show sol-gel phase transition and thus trigger drug release in response to external stimuli, are the most investigated. In-situ hydrogels are providing such ‘sensor’ properties and can undergo reversible sol-gel phase transitions upon changes in the environmental condition. These “intelligent” or “smart” polymers play important role in drug delivery since they may dictate not only where a drug is delivered, but also when and with which interval it is released.15
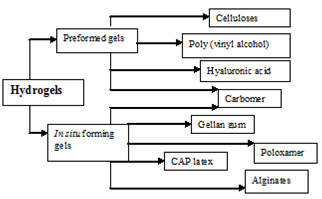
Figure 2: Classification of hydrogels.
A polymer used to prepare in situ gels should have following charterstics16
1. It should be biocompatible.
2. It should be capable of adherence to mucus.
3. It should have pseudoplastic behaviour.
4. It should have good tolerance and optical clarity.
5. It should influence the tear behaviour.
6. The polymer should be capable of decreasing the viscosity with increasing shear rate there by offering lowered viscosity during blinking and stability of the tear film during fixation.
7. The first use of gels for medical applications was presented by Wichterle and Lim in 1960. in manufacturing of soft contact lenses and implant materials from hydroxyethyl methacrylate polymers.17
METHOD FOR PREPARATION:
There are many methods have been employed to cause reversible sol-gel phase transition in cul de sac and some of these methods are change in temperature, pH and electrolyte composition.18, 19,20
Thermo reversible in situ gels:
These hydrogels are liquid at room temperature (20 –25°C) and undergo gelation when in contact with body fluids (35–37°C), due to an increase in temperature.21 Example: Poloxamers, cellulose derivatives, and xyloglucan
The Poloxamers (Figure 3) consist of more than 30 different non-ionic surface active agents. These polymers are ABA-type tri block copolymers composed of polyethylene oxide (PEO) (A) and polypropylene oxide (PPO) units (B). The Poloxamer series covers a range of liquids, pastes, and solids, with molecular weights and ethylene oxide–propylene oxide weight ratios varying from 1100 to 14,000 and 1:9 to 8:2, respectively.22
Poloxamers, commercially available as Pluronic®, are the most commonly used thermal setting polymers in ophthalmology. They are formed by central hydrophobic part (polyoxypropylene) surrounded by hydrophilic part (ethylene oxide). Depending on the ratio and the distribution along the chain of the hydrophobic and hydrophilic subunits several molecular weights are available, leading to different gelation properties. Pluronic F-127, which gives colorless and transparent gels, is the most commonly used polymer in pharmaceutical technology.
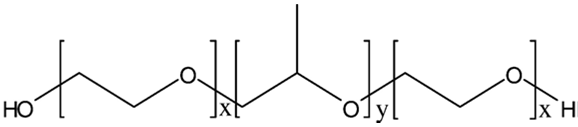
Figure 3: PEO-PPO-PEO (Poloxamer).
The gelation mechanism of Poloxamer (Figure4) solutions has been investigated extensively, but is still being debated. Ultrasonic velocity, light-scattering and smallangle neutron scattering measurements of aqueous Poloxamer solutions have clearly indicated a micellar mode of association. Micelle formation occurs at the critical micellization temperature as a result of PPO block dehydration. With increasing temperature, micellization becomes more important, and at a definite point, micelles come into contact and no longer move. In addition, the formation of highly ordered structures, such as cubic crystalline phase, has been proposed as the driving force for gel formation, but this hypothesis has been questioned recently. Thus, packing of micelles and micelle entanglements may be possible mechanisms of Poloxamer solution gelation with increased of temperature. Furthermore, it has suggested that intramolecular hydrogen bonds might promote gelation.The Poloxamers are reported to be well tolerated and non-toxic even though large amounts (25-30%) of polymers are required to obtained a suitable gel.22, 23
Three principal mechanisms have been proposed to explain the liquid-gel phase transition after an increase in temperature, including:-
1. Gradual desolvation of the polymer.
2. Increased micellar aggregation and
3. The increased entanglement of the polymeric network.
Despite all the promising results obtained with thermo reversible gels, there remains an important drawback associated with their use; the risk of gelation before administration by increase in ambient temperature during packing or storage.
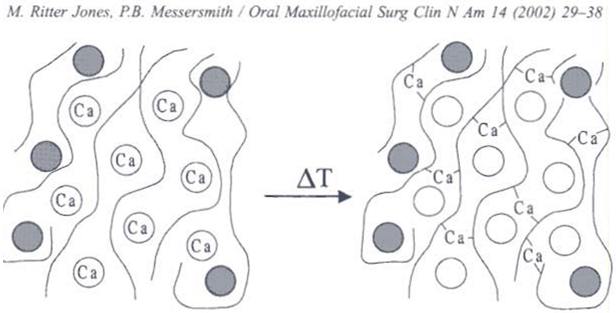
Figure 4: in situ gel formation due to change in temperature.
Thermo reversible gels can be prepared with naturally occurring polymers. Most natural polymer aqueous solutions form a gel phase when their temperature is lowered. Classic examples of natural polymers exhibiting a sol–gel transition include gelatin and carrageenan. At elevated temperatures; these polymers adopt a random coil conformation in solution. Upon cooling, a continuous network is formed by partial helix formation.24
Cellulose derivatives also cause gelation Eg: Methylcellulose and hydroxypropylmethylcellulose (HPMC) are typical examples of such polymers. Methylcellulose solutions transform into opaque gels between 40 and 50 °C, whereas HPMC shows phase transition between 75 and 90°C. These phase transition temperatures can be lowered by chemical or physical modifications. For Eg: NaCl decreases the transition temperature of methylcellulose solutions to 32–34°C.
Xyloglucan a polysaccharide derived from tamarind seed, forms thermo responsive gels in water, under certain conditions. Xyloglucan is composed of a (1-4)-β-D glucan backbone chain (GLU) which presents (1-6)-α-D-xylose branches (XYL) partially substituted by (1-2)-β-Dgalactoxylose (GAL). Tamarind seed xyloglucan is composed of three units of xyloglucan oligomers with heptasaccharide, octasaccharide and nonasaccharide, which differ in the number of galactose side chains. When xyloglucan is partially degraded by β-galactosidase, the resultant product exhibits thermally reversible gelation in dilute aqueous solutions. Such behavior does not occur with native xyloglucan. Gelation is only possible when the galactose removal ratio exceeds 35%. The transition temperature is inversely related to polymer concentration and the galactose removal ratio. For Eg: the sol–gel transition of xyloglucan was shown to decrease from 40 to 5°C when the galactose removal ratio increased from 35 to 58%.Xyloglucan is approved for use as a food additive. However, its relatively low transition temperature (22–27°C) makes handling at room temperature problematic.25,26, 27
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
pH sensitive in situ gels:
Gelling of the solution is triggered by a change in the pH. Eg: Cellulose acetate phthalate (CAP) latex and its derivatives such as Carbomer (Figure 5) are used. Cellulose acetate derivatives are the only polymer known to have a buffer capacity that is low enough to gel effectively in the cul-de-sac of the eye. The pH change of about 2.8 units after instillation of the native formulation (pH 4.4) into the tear film leads to an almost instantaneous transformation of the highly fluid latex into viscous gel. First preliminary investigations of pH sensitive latexes for ophthalmic administration began in early 1980s and have been extensively studied by Boye.28, 29
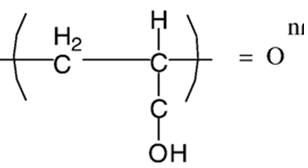
Figure 5: structure of Carbomer.HODS
Cellulose acetate phthalate latex is a polymer with potentially useful properties for sustained drug delivery to the eye because latex is a free running solution at a Ph of 4.4, which undergoes coagulation when the pH is raised by the tear fluid to pH 7.4. But the low pH of the preparation can elicit discomfort in some patients.30 The poly acrylic acid and its lightly cross-linked commercial forms (Polycarbophil and Carbopol) exhibit the strongest mucoadhesion Carbomer (Carbopol) a cross-linked acrylic acid polymer (PAA) also shows pH induced phase transition as the pH is raised above its pKa of about 5.5. Different grades of Carbopol are available. The manufacturer states that Carbopol 934 gel has the lowest cross-linking density, while Carbopol 981 intermediate and Carbopol 940 have the highest, higher the cross linking ability more stiff is the gel formed. The amount of PAA required to form stiff gel upon instillation in the eye is not easily neutralized by the buffering action of tear fluid and as the concentration of Carbopol increases in the vehicle, its acidic nature may cause stimulation to the eye tissues. In order to reduce the total polymer content and improve the gelling properties, an ocular drug delivery system based on a combination of Carbopol and a suitable viscosity enhancing polymer Eg: HPMC or MC allows a reduction in the PAA concentration without comprising the in situ gelling properties.31, 31, 33, 34
All pH-sensitive polymers contain pendant acidic or basic groups that either accept or release protons in response to changes in environmental pH. The polymers with a large number of ionizable groups are known as polyelectrolytes. Swelling of hydrogel increases as the external pH, increases in the case of weakly acidic (anionic) groups, but decreases if polymer contains weakly basic (cationic) groups.
Some of examples of that are delivered by pH sensitive method include ciprofloxacin, Indomethacin, Gatifloxacin.35,36,37
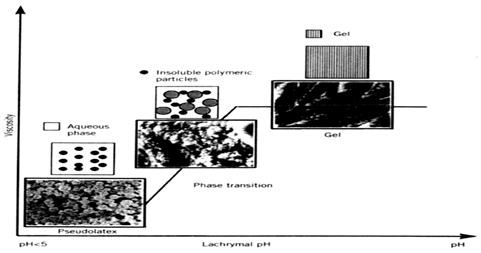
Figure 6: Schematic representation of pH dependent in situ gels.
Ion sensitive in situ gels:
Polymers may undergo phase transition in presence of various ions. Some of the polysaccharides fall into the class of ion-sensitive ones. While k-carrageenan forms rigid, brittle gels in reply of small amount of K+, icarrageenan forms elastic gels mainly in the presence of Ca2+. Gellan gum commercially available as Gelrite® is an anionic polysaccharide that undergoes in situ gelling in the presence of mono and divalent cations, including Ca2+, Mg2+, K+ and Na+. Gelation of the low-methoxy pectins can be caused by divalent cations, especially Ca2+. Likewise, alginic acid undergoes gelation in presence of divalent/polyvalent cations.38 Gellan gum (Gelrite) is a linear, anionic hetero polysaccharide secreted by the microbe Sphingomonas elodea (formerly known as Pseudomonas elodea). The polysaccharide can be produced by aerobic fermentation and then isolated from the fermentation broth by alcoholIprecipitation. The polymer backbone consists of glucose, glucuronic acid, and rhamnose in the molar ratio 2:1:1. These are linked together to give a tetra saccharide repeat unit .The native polysaccharide is partially esterified with L-glycerate and acetate, but the commercial product Gelrite has been completely deesterified by alkali treatment. Gelrite® (deacetylated gellan gum) is one of the most interesting in situ gelling polymers that has been tested since it seems to perform very well in humans. Gelrite has been granted regulatory approval as pharmaceutical excipient and is marketed by Merck in a controlled-release glaucoma formulation called Blocarden® Depot (Timoptic®). Formulations with the Gelrite can be administered to ocular mucosa as a low viscosity solution. On contact with cations in tear fluid the formulation will form a clear gel. This is caused by cross linking of the negatively charged polysaccharide helices by monovalent and divalent cations (Na+, K+, Ca+). Several models have been presented to explain gellan gum gelation. 39, 40, 41, 42N
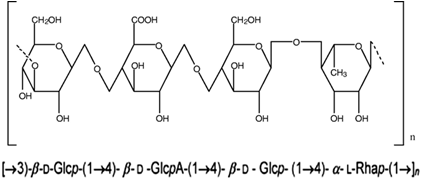
Figure 7: Structure of deacetylated gellan gum.
Mechanism involved in sol to gel transistion by gelrite is as follows, in an ion free aqueous medium, Gelrite forms double helices at room temperature. This solution has a viscosity close to that of water and the helices are only weakly associated with each other (by van der Waals attraction). When gel-promoting cations are present, some of the helices associate into cation-mediated aggregates, which cross-link the polymer. On heating the polysaccharide in an ion free environment, the polysaccharide becomes a disordered coil. However, on heating a sample with cations present, the non aggregated helices melt out first, and the aggregated helices melt out at a higher temperature in a second transition. The divalent ions such as magnesium or calcium were superior to monovalent cations in promoting the gelation of the polysaccharide. However the concentration of sodium in tears (2.6 g/L) is quite sufficient to induce the gelation. Corneal contact time of formulations based on gellan gum has been investigated using two main methods, which are fluorometry and γ- scintigraphy. Both techniques have demonstrated improved residence times with Gelrite when compared with saline or various commercial solutions. Gelrite has also provided corneal residence time superior to those ofother hydrogel preparations based on polymers such as cellulosic derivatives or xanthan gum. 43, 44, 45
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Alginates Being a family of unbranched binary copolymers, alginates consist of (1→4) linked β-Dmannuronic acid (M) and α-L guluronic acid (G) residues of widely varying composition and sequence. By partial acid hydrolysis, alginate was separated into three fractions. Two of these contained almost homopolymeric molecules of G and M, respectively, while a third fraction consisted of nearly equal proportions of both monomers and was shown to contain a large number of MG dimer residues. It was concluded that alginate could be regarded as a true block copolymer composed of homo polymeric regions of M and G, termed M- and G-blocks, respectively, interspersed with regions of alternating structure. It was further shown that alginates have no regular repeating unit and that the distribution of the monomers along the polymer chain could not be described by Bernoullian statistics. Knowledge of the monomeric composition is hence not sufficient to determine the sequential structure of alginates. Alginate with a high guluronic acid content will improve the gelling properties and reduce the total polymer to be introduced into the eye. The alginate forms 3-dimensional ionotropic hydrogel matrices, generally by the preferential interaction of calcium ions with the G moieties resulting in the formation of in homogeneous gel. Eg: Sodium alginate. 46, 47, 48
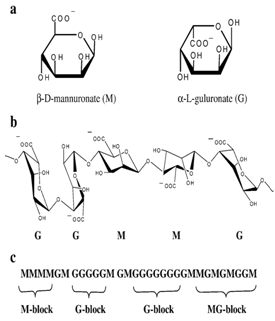
Figure 8: Structural characteristics of alginates: (a) alginate monomers (b) chain conformation and (c) block distribution.

Figure 9: Sol-gel transformation of polymers at formulation and physiological conditions of the eye.
CONCLUSION:
In conclusion, in situ gels offer the primary requirement of a successful controlled release product that is increasing patient compliance. Exploitation of polymeric in situ gels for controlled release of various drugs provides a number of advantages over conventional dosage forms. Sustained and prolonged release of the drug, good stability and biocompatibility characteristics make the in situ gel dosage forms very reliable. In situ activated gel forming systems seem to be preferred as they can be administered in drop form and create significantly less problems with vision. Moreover, they provide good sustained release properties. Over the last decades, an impressive number of novel temperature, pH, and ion induced in-situ forming solutions have been described in the literature. Each system has its own advantages and drawbacks. The choice of a particular hydrogel depends on its intrinsic properties and envisaged therapeutic use. Future use of biodegradable and water soluble polymers for the in situ gel formulations can make them more acceptable and excellent drug delivery systems.
REFERENCES:
1. Rathore KS, Nema RK, Ishibashi Tejraj, Yokoi N, Born JA, Tiffany MJ, Komuro A. Review on Ocular inserts International Journal of Pharm Tech Research 1(2); 2009: 164-169.
2. Rathore KS, Nema RK. Formulation and evaluation of ophthalmic films for timolol maleate. Planta indica4; 2008:49-50.
3. Keister JC, Cooper ER, Missel PJ, Lang JC, Hager DF. Limits on optimizing ocular drug delivery Journal of Pharmaceutical Sciences 80; 1991: 50-53.
4. J.L. Greaves, C.G. Wilson, A.T. Birmingham. Assessment of the precorneal residence of an ophthalmic ointment in healthy subjects. British journal of clinical pharmacology 35; 1993:188–192.
5. Mazor Z, Ticho U, Rehany U, Rose L, Piloplex.A new longacting pilocarpine polymer salt. B:comparative study of the visual effects of pilocarpine and Piloplex eye drops British journal of ophthalmology 63; 1979: 58–61.
6. Gangrade N.K, Gaddipati N.B, Ganesan M.G, Reddy I.K, Ocular Therapeutics and Drug Delivery, Technomic Publishing, Lanchester.1996; 377–403.
7. Olejnik O, Mitra A.K, Novel Control Drug Delivery Systems, Marcel Dekker, New York.1993; 117–197.
8. Sanzgiri Y.D, Maschi S, Crescenzi V, Callegaro L, Topp E.M, Stella V.J. Gellan-based systems for Ophthalmic sustained delivery of methyl prednisolone. Journal of Controlled Release 26; 1993:195–201.
9. Durrani A.M,Davies N.M,Thomas M, Kellaway I.W. Pilocarpine bioavailability from a mucoadhesive liposomal ophthalmic drug delivery system. International journal of pharmaceutics 88; 1992: 409–415.
10. Fitzgerald P, Hadgraft J., Kreuter J, Wilson C.G. A gamma scintigraphic evaluation of microparticulate ophthalmic delivery systems. Liposomes and nanoparticles. International journal of pharmaceutics 40; 1987: 81–84.
11. Divyesh H. Shastri, Lakshmanbhai D. Patel. A Novel Alternative to Ocular Drug Delivery System: Hydrogel International journal of pharmaceutical research 2(1); 2010:1-13.
12. Bourlais C.L, Acar L, Zia H, Sado P.A, Needham T, Leverge R. Ophthalmic Drug Delivery Systems-Recent advances progress in retinal and eye research 17;1998:33–58.
13. Robinson JR. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. Journal of pharmaceutical sciences 64; 1975:1312-1316.
14. Felt O, Baeyens V, Zignani M, Buri P,Gurny R. Mucosal drug delivery ocular Encyclopaedia of controlled drug delivery, volume 2, University of Geneva, Geneva, Switzerland 1999: 605–622.
15.Soppinath KS, Aminabhavi, TM, Dave AM, Kumar SG,Rudzinski WE. Stimulus responsive“smart” hydrogels as novel drug delivery systems. Drug Development and Industrial Pharmacy 28; 2002: 957-74.
16. Van M. In Biopharmaceutics of ocular drug delivery, Ed. P. Edman, CRC press, Boca Raton, Fla 1993:27-42.
17. Wichterle O, Lim D. Hydrophilic gels for biological use Nature 185; 1960:117-118.
18. Miller S.C, Donovan M.D. Effect of Poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. International journal of pharmaceutics 12; 1982:147– 152.
19. Gurny R, Boye T, Ibrahim H. Ocular therapy with nanoparticulate systems for controlled drug delivery. Journal of Controlled Release 2; 1985: 353–361.
20. Moorhouse R, Colegrove G.T, Sandford P.A, Baird J.K, Kang K.S. A new gel forming polysaccharide, Solution Properties of Polysaccharides. ACS Symposium series, Washington-DC. 1981; 111–124.
21. Hoffman A.S, Afrassiabi A, Dong A. Thermally reversible hydrogels: II. Delivery and selective removal of substances from aqueous solutions. Journal of Controlled Release 4; 1986: 213–222.
22. Gariepy E.R, Leroux J.C. In situ-forming hydrogels review of temperature-sensitive systems. European journal of pharmaceutics and bio pharmaceutics 58; 2004: 409–426.
23. Cabana A, Ait-Kadi A, Juhasz J. Study of the gelation process of polyethylene oxide polypropylene oxide–polyethylene oxide copolymer (Poloxamer 407) aqueous solutions.Journal of colloid and interface sciences 190; 1997: 307–312.
24. Harrington W.F, Von Hippel W.F. The structure of collagen and gelatin. Advances in Protein Chemistry 1; 1961: 1–138.
25. Shirakawa M, Yamatoya K, Nishinari K. Tailoring xyloglucan properties using an enzyme. Food Hydrocolloids 22; 1998: 25–28.
26. Miyazaki S, Suisha F, Kawasaki N., Shirakawa M, Yamatoya K, Attwood D. Thermally reversible xyloglucan Volume 7, Issue 1, March – April 2011
27. Gariepy E.R, Leroux J.C.In situ-forming hydrogels—reviews of temperature-sensitive systems. European journal of pharmaceutics and bio pharmaceutics 58; 2004: 409–426.
28. Lin HR, Sung KC. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. Journal of Controlled Release 69; 2000: 379.
29. Boye T, Gurny R, Ibrahim H. Ocular therapy with nanoparticulate systems for controlled drug delivery. Journal of Controlled Release 2; 1985:353-361.
30. Le Bourlais CA, Treupel-Acar L, Rhodes Ct, Sado PA, Leverge R. New ophthalmic drug delivery system. Drug Development and Industrial Pharmacy. 21; 1995:19-59.
31. Hui HW, Robinson JR. Ocular drug delivery of progesterone using of bioadhesion polymer. International Journal of Pharmaceutical Sciences 26; 1985:203-213.
32. Davis NM, Farr SJ, Hadgraft J, Kellaway IW. Evaluation of mucoadhesive polymers in ocular drug delivery: part 1, viscous solution. Pharma Research. 8(8); 1991:1039- 1043.
33. Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. Journal of Controlled Release73; 2001:205-11.
34. Kumar S, Haglund BO, Himmelstein KJ. In situ-forming gels for ophthalmic drug delivery. Journal of ocular pharmacology 10; 1994:47- 56.
35. Mohan EC, Jagan Mohan K, Venkatesham A. Preparation and evaluation of in situ gels for ocular drug delivery. Journal of pharmaceutical research 2(6);2009: 1089- 94.
36. Thilak kumar M, Bharathi D, Balasubramaniam J, Kant S, Pandit J.K. pH induced in situ gelling of Indometacin for Sustained ocular delivery. Indian Journal of Pharmaceutical Sciences 67(3); 2005:327-33.
37. Doijad RC, Manvi FV, Malleswara VSN, Prajakta Alase. Sustained ophthalmic delivery of Gatifloxacin from in Situ Gelling System. Indian Journal of Pharmaceutical Sciences 68(6); 2006: 809-814.
38. Bhardwaj TR, Kanwar M, Lal R, Gupta A. Natural gums and modified natural gums as sustained release carriers. Drug Development and Industrial Pharmacy 26; 2000:1025-38.
39. Jansson P.E, Lindberg B. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseuomonas elodea. Carbohydrate Research 124;1983: 135–139.
40. Kuo M.S, Mort A.J, Dell A. Identification and location of L-glycerate, an unusual acyl substituent in gellan gum. Carbohydrate Research 156; 1986: 173–187.
41. Morris V.J, Stephen A.M. Bacterial polysaccharides, in food polysaccharides and their application, Marcel Dekker, New York, 1995; 341–375.
42. Rozier A, Mazuel C, Grove J, Plazonnet B. Gelrite®: a novel ion activated in situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. International journal of pharmaceutics 57; 1989: 163–168.
43. Kang K.S, Veeder G.T, P.J Mirrasoul P.J, Kaneko T, Cottrell I.W, Agarlike. Polysaccharide produced by a Pseudomonas species: production and basic properties. Applied and Environmental Microbiology 43; 1982: 1086–1091.
44. Maurice D.M, Srinivas S.P. use of fluorometry in assessing the efficacy of a cation-sensitive gel as an ophthalmic vehicle: comparison with scintigraphy. Journal of Pharmaceutical Sciences 81; 1992: 615–618.
45. Meseguer G, Gurny R, Buri P, Rozier A, Plazonnet B. Gamma scintigraphic study of precorneal drainage and assessment of miotic response in rabbits of various ophthalmic formulations containing pilocarpine. International journal of pharmaceutics 95; 1993:229–234.
46. Haug A, Larsen B, Smidsrod O.A study of the constitution of alginic acid by partial hydrolysis Acta Chemica Scandinavica 20; 1966: 183–190.
47. Painter T.J, Smidsrod O, Haug A. A computer study of the changes in composition distribution occurring during random depolymerisation of a binary linear heteropolysaccharide. Acta Chemica Scandinavica 22; 1968: 1637–1648.
48. Larsen B., Smidsrod O,. Painter T.J, Haug A. Calculation of the nearest neighbor frequencies in fragments of alginate from the yields of free monomers after partial hydrolysis. Acta Chemica Scandinavica 24; 1970: 726–728.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









