 About Authors:
About Authors:
Bhawana Kapoor*1, Vishnukant Rai2, Sonu Sharma3
1Seth G.L. Bihani S.D. College of Technical Education, Sriganganagar, Rajasthan, India
2Shri Ramnath Singh College of Pharmacy, Gormi, Bhind, M.P., India
3NIMS Institute of Pharmacy, Jaipur, Rajasthan, India
ABSTRACT
Chromatography, although primarily a separation technique, is mostly employed in chemical analysis in which High-performance liquid chromatography (HPLC) is an extremely versatile technique where analytes are separated by passage through a column packed with micrometer-sized particles. Now a day reversed-phase chromatography is the most commonly used separation technique in HPLC. The reasons for this include the simplicity, versatility, and scope of the reversed-phase method as it is able to handle compounds of a diverse polarity and molecular mass. This review covers the importance of RP-HPLC in analytical method development and their strategies along with brief knowledge of critical chromatographic parameters need to be optimized for an efficient method development.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1237
1. INTRODUCTION
Chromatography is probably the most powerful analytical technique available to the modern chemist. Its power arises from its capacity to determine quantitatively many individual components present in mixture by single analytical procedure1-2. High-performance liquid chromatography (HPLC) is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture3. It is an extremely versatile technique where analytes are separated by passage through a column packed with micrometer-sized particles4. Most of the drugs in multicomponent dosage forms can be analyzed by HPLC method because of the several advantages like rapidity, specificity, accuracy, precision and ease of automation in this method. There are different modes of separation in HPLC like normal phase mode, reversed phase mode, reverse phase ion pair chromatography, affinity chromatography and size exclusion chromatography5. Now a day reversed-phase chromatography is the most commonly used separation technique in HPLC due to its broad application range. It is estimated that over 65% (possibly up to 90%) of all HPLC separations are carried out in the reversed-phase mode. The reasons for this include the simplicity, versatility, and scope of the reversed-phase method as it is able to handle compounds of a diverse polarity and molecular mass6-8.
1.1. Theory of reversed phase chromatography
Reversed phase chromatography has found both analytical and preparative applications in the area of biochemical separation and purification. Molecules that possess some degree of hydrophobic character can be separated by reversed phase chromatography with excellent recovery and resolution9. Reversed-phase chromatography is performed on columns where the stationary phase surface is less polar than the mobile phase. Retention in reversed-phase liquid chromatography (RPLC) occurs by nonspecific hydrophobic interactions of the solute with the stationary phase. The near-universal application of reversed-phase chromatography stems from the fact that virtually all organic molecules have hydrophobic regions in their structures and are capable of interacting with the stationary phase. Reversed-phase chromatography is thus ideally suited to separating the components of oligomers or homologues. Since the mobile phase in reversed-phase chromatography is polar and generally contains water, the method is ideally suited to the separation of polar molecules that are either insoluble in organic solvents or bind too strongly to solid adsorbents for normal elution. Many samples with a biological origin fall into this category8.
1.2. RP-HPLC separation mechanism
In general, polarity is the primary characteristics of compounds (chemicals) that can be used to create RP-HPLC separations in a mixture. All components in a mixture have a unique behavioral characteristic related to their molecular structure. They can be described as being “Polar” or “Non-Polar”, with a range of polarities between the most polar and most non-polar. The polarity characteristic of chemicals is mainly used to build chromatographic “Retention Mechanisms” that are used to create many HPLC separations. Because of polarity, there can be either “Attraction” or “Repulsion” between two chemical species. A simple rule describes this behavior for polarity basedretention mechanisms: “like attracts like and opposites is not attracted when we use polarity”. For effective chromatographic separation system, making the mobile phase and the stationary phase with different polarities creates a “Competition” for the different sample compounds. Since the compounds in the sample have different polarities, compounds which have the same polarity as the stationary phase (column packing material) will slow down (because they are attracted to the particles). Any compounds that have the same polarity as the moving mobile phase (attracted) will move along at a faster speed. Separation is made effective by changing the relative attractions (and therefore the speeds) of each compound10.
[adsense:468x15:2204050025]
2. ANALYTICAL METHOD DEVELOPMENT USING RP-HPLC
Methods of analysis are routinely developed, improved, validated, collaboratively studied and applied. Compilations of these developed methods then appear in large compendia such as USP, BP and IP, etc. In most cases as desired separation can be achieved easily with only a few experiments. In other cases a considerable amount of experimentation may be needed. However, a good method development strategy should require only as many experimental runs as are necessary to achieve the desired final result(s). The development of a method of analysis is usually based on prior art or existing literature using almost the same or similar experimentation. The development of any new or improved method usually tailors existing approaches and instrumentation to the current analyte, as well as to the final need or requirement of the method. Method development usually requires selecting the method requirements and deciding on what type of instrumentation to utilize and why. In the HPLC method development stage, decisions regarding choice of column, mobile phase, detectors, and method quantitation must be considered. So development involves a consideration of all the parameters pertaining to any method. Therefore, development of a new HPLC method involves selection of best mobile phase, best detector, best column, column length, stationary phase and best internal diameter for the column11-12. The analytical strategy for HPLC method development contains a number of steps13, as shown in figure 1.
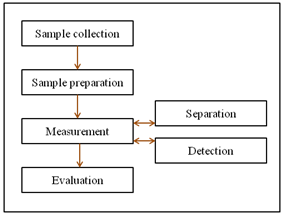
Figure 1: A typical strategy for HPLC method development
2.1. Sample collection and preparation
Sample preparation is an essential part of HPLC analysis, intended to provide a reproducible and homogenous solution that is suitable for injection onto the column. The aim of sample preparation is a sample aliquot that,
* Is relatively free of interferences,
* Will not damage the column, and
* Is compatible with the intended HPLC method that is, the sample solvent will dissolve in the mobile phase without affecting sample retention or resolution12.
Sample preparation begins at the point of collection, extends to sample injection onto the HPLC column and encompasses the various operations summarized in table 5. All of these operations form an important part of sample preparation and have a critical effect on the accuracy, precision, and convenience of the final method12.
Table 5: Sample pretreatment options
|
S.No. |
Option |
Comment |
|
1. |
Sample collection |
Obtain representative sample using statistically valid processes. |
|
2. |
Sample storage and preservation |
Use appropriate inert, tightly sealed containers; be especially careful with volatile, unstable, or reactive materials; biological samples may require freezing. |
|
3. |
Preliminary sample processing |
Sample must be in a form for more efficient sample pretreatment (e.g., drying, sieving, grinding, etc.); finer dispersed samples are easier to dissolve or extract. |
|
4. |
Weighing or volumetric dilution |
Take necessary precautions for reactive, unstable, or biological materials; for dilution, use calibrated volumetric glasswares. |
|
5. |
Alternative sample processing methods |
Solvent replacement, desalting, evaporation, freeze drying, etc. |
|
6. |
Removal of particulates |
Filtration, solid-phase extraction, centrifugation. |
|
7. |
Sample extraction |
Different methods used for liquid samples and solid samples. |
|
8. |
Derivatization |
Used mainly to enhance analyte detection; sometimes used to improve separation. |
2.2. Measurement
The measurement of a given analyte can often be divided into a separation step and a detection step.
2.3. Separation
Analytes in a mixture should preferably be separated prior to detection. Simple LC consists of a column with a fritted bottom containing the stationary phase in equilibrium with a solvent. The mixture to be separated is loaded on to the top of the column followed by more solvent. The different components in the column pass at different rates due to difference in their partitioning behavior between mobile liquid phase and stationary phase13, 14.
2.4. Detection
A large number of LC detectors have been developed over the past thirty years based on a variety of different sensing principles for detecting the analytes after the chromatographic separations. However, only about twelve of them can be used effectively for LC analysis and, of those twelve, only four are in common use. The four dominant detectors used in LC analysis are the UV detector (fixed and variable wavelength), the electrical conductivity detector, the fluorescence detector and the refractive index detector. These detectors are employed in over 95% of all LC analytical applications. The choice of detector depends on the sample and the purpose of the analysis15.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3. METHOD OPTIMIZATION
The methods developed will then be optimized according to the goal of separation, i.e. isolation of purified material or routine quantitative analysis.During the optimization stage, the initial sets of conditions that have evolved from the first stages of development are improved or maximized in terms of resolution and peak shape, plate counts asymmetry, capacity, elution time, detection limits, limit of quantitation, and overall ability to quantify the specific analyte of interest. Stationary phase, mobile phase and detectors are the critical parameters requiring optimization.
3.1. Critical parameters: Selection and optimization
Before proceeding for the method development it is always important to have thorough knowledge about the sample, which can provide valuable clues for the best choice of initial conditions for an HPLC separation.The sample must be analyzed for its major components and any contaminants. The chemical composition of the sample can provide valuable clues for the best choice of initial conditions for an HPLC separation. Various important information concerning sample composition and properties include number of compounds present, chemical structures, molecular weight, pKa values, UV spectra of compounds, concentration range of compounds in sample and sample solubility11-12.
3.1.1. Classifying the sample
The first step in method development is to characterize the sample as regular or spherical. Regular samples are a mixture of small molecules (<2000 Daltons) that can be separated using more or less standardized starting conditions. Separations in regular samples respond in predictable fashion to change changes in solvent strength (%B) and type (Acetonitrile, methanol) or temperature. A 10% decrease in %B increases retention by about threefold, and selectivity usually changes as either %B or solvent type is varied. It is possible to separate many regular samples just by varying solvent strength and type. Therefore, RPC method development for all regular samples (both neutral and ionic) can be carried out initially in the same way11-12. For regular samples, the preferred experimental conditions for the initial HPLC separation are shown in table 1.
Table 1: Experimental conditions for initial HPLC separation of regular samples
|
Parameter(s) |
Specifications |
|
Column |
15 cm ´ 4.6 mm (I.D), 250 mm ´ 4.6 mm |
|
Particle size of packing |
5 µm, 10 µm |
|
Stationary phase |
RP-C18, RP-C8 |
|
Mobile phase |
Buffer : methanol : acetonitrile |
|
Buffer |
25-50 mM K2HPO4, pH 2-3 |
|
Additives |
Amines (triethylamine, diethylamine), Ion pair reagents are not tried initially |
|
Flow Rate |
1-2 mL/min |
|
Temperature |
Ambient |
3.1.2. The column/Stationary phase
Selection of the stationary phase/column is the first and the most important step in method development. The development of a rugged and reproducible method is impossible without the availability of a stable, high performance column. To avoid problems from irreproducible sample retention during method development, it is important that columns be stable and reproducible. A C8 or C18 column made from specially purified, less acidic silica and designed specifically for the separation of basic compounds is generally suitable for all samples and is strongly recommended6,12-13,16-17. Some important factors need to be considered while selecting column in RP-HPLC are summarized in table 2.
Table 2:Factors affecting column efficiency
|
Factor(s) |
Effect on column efficiency |
|
Column length |
*Choose longer columns for enhanced resolution *Choose shorter column for shorter analysis time, lower back pressure and fast equilibration and less solvent consumption |
|
Column internal diameter |
*Choose wider diameter column for greater sample loading *Choose narrow column for more sensitive and reduced mobile phase consumption |
|
Particle shape |
*Choose spherical particles for lower back pressure, column stability and greater stability *Choose irregular particles when high surface area and high capacity is required |
|
Particle size |
*Choose smaller particle (3-4 µm) for complex mixture with similar components *Choose larger particle (5-10 µm) for sample with structurally different compounds *Choose very large particle (15-20 µm) for preparative separation |
|
Pore size |
*Choose a pore size of 150?or less for sample with molecular weight less than 2000 *Choose a pore size of 300?or less for sample with molecular weight greater than 2000 |
|
Surface area |
*Choose end capped packing to eliminate unpredictable secondary interaction with the base materials *Choose non-end capped phase for selectivity differences for polar compounds by controlling secondary interaction |
|
Carbon load |
*Choose high carbon loads for greater column capacities and resolution *Choose low carbon loads for fast analysis |
The column should provide,
* Reasonable resolution in initial experiments,
* Short run time,
* An acceptable pressure drop for different mobile phases12.
The column is selected depending on the nature of the solute and the information about the analyte. Reversed phase mode of chromatography facilitates a wide range of columns like dimethyl silane (C2), butylsilane (C4), octylsilane (C8), octadecylslane (C18), base deactivated silane (C18) BDS phenyl, cyanopropyl (CN), nitro, amino, etc. Generally longer columns provide better separation due to higher theoretical plate numbers. As the particle size decreases the surface area available for coating increases. Columns with 5-µm particle size give the best compromise of efficiency, reproducibility and reliability.
3.1.3. The mobile phase
The choice of suitable mobile phase is vital in HPLC as a successful chromatographic separation depends upon differences in the interaction of the solutes with the mobile phase and the stationary phase. The eluting power of the mobile phase is determined by its overall polarity, the polarity of the stationary phase and the nature of the sample components. For reverse phase separations, eluting power decreases with increasing solvent polarity. Many of the common solvents used in the HPLC are flammable and some are toxic, so it is advisable for HPLC instrumentation to be used in well ventilated laboratory. Special grades of solvents are available for HPLC which have been carefully purified to remove UV-absorbing impurities and any particulate matter. It is also important to remove dissolved air or suspended air bubbles which can cause problems affecting the detector and pump. Variations of the eluant composition, type of organic modifier, pH and buffer concentration form a valuable set of variables for successful development of a separation method12,18. Buffer strength & pH, buffer composition, mobile phase polarity, viscosity, selectivity, absorbance, and additives are some of the parameters, which shall be taken into consideration while selecting and optimizing the mobile phase.
3.1.4. Buffer and its strength
In many cases, the colloquial term used for the mobile phases in reversed phase chromatography is “buffer”. Buffer and its strength play an important role in deciding the peak symmetries and separations. Some of the most, commonly employed buffers are:
* Phosphate buffers prepared using salts like KH2PO4, K2HPO4, NaH2PO4, Na2HPO4, etc
* Phosphoric acid buffers prepared using H3PO4
* Acetate buffers-Ammonium acetate, Sodium acetate, etc
* Acetic acid buffers prepared using CH3COOH
The retention times also depend on the molar strengths of the buffer. Molar strength is increasingly proportional to retention times.
3.1.5. Buffer pH
pH plays an important role in achieving the chromatographic separations as it controls the elution properties by controlling the ionization characteristics. Experiments were conducted using buffers having different pH to obtain the required separations. For example the influence of the pH of the buffer on the retention of diuretics was studied. When the buffer pH was changed from 8 to 3 a drastic change in the selectivity was obtained. For most of the compounds, the retention was higher at pH 3. This could be explained by the fact that most compounds have acidic groups in their structure that are protonated at low pH, thereby increasing the compounds’ hydrophobicities. The opposite was observed for triamterene, a compound with several amine groups, that carries a positive charge at pH 3.
It is important to maintain the pH of the mobile phase in the range of 2.0 to 8.0 as most columns does not withstand to the pH which are outside this range. This is due to the fact that the siloxane linkage area cleaved below pH 2.0; while at pH valued above 8.0 silica may dissolve12,19.
3.1.6. Mobile phase composition
Most chromatographic separations can be achieved by choosing the optimum mobile phase composition. This is due to that fact that fairly large amount of selectivity can be achieved by choosing the qualitative and quantitative composition of aqueous and organic portions. Most widely used solvents in reverse phase chromatography are methanol and acetonitrile. Experiments are required to be conducted with mobile phases having buffers with different pH and different organic phases to check for the best separations between the impurities. In RP-HPLC the organic part of eluant is considered the strong solvent. Increasing the fraction of organic solvent increases the solvent strength and allows for elution of the species in a mixture, resulting in smaller analyte retention factors or retention volumes18.
3.1.7. Absorbance
An UV-visible detector is based on the principle of absorption of UV visible light from the effluent emerging out of the column and passed through a photocell placed in the radiation beam. UV detector is generally suitable for gradient elution work. Most compounds adsorb UV light in the range of 200-350 A°. The mobile phase used should not interfere in the peak pattern of the desired compound hence it should not absorb at the detection wavelength employed20.
3.1.8. Polarity and Selectivity
Separation is affected by the polarity and selectivity of mobile phase solvents. More polar solvents cause increased retention in RPC or reduce retention in NPC. The polarity values range from P´=0 for a non-polar solvent (like pentane) to P´= 10.2 for the very polar solvent (like water). In adsorption and partition chromatography the selectivity, α, can be somewhat controlled by changing the composition of the mobile phase, while maintaining constant eluant strength. In general, if an eluant prepared from two relatively weak solvents gives inadequate α, another eluant of equal strength prepared by adding a small amount of a strong solvent to the weak solvent, will most likely improve selectivity.
3.1.9. Viscosity
Solvent of lowest possible viscosity should be used to minimize separation time. An added advantage of low viscosity is that high efficiency theoretical plate (HETP) values are usually lower than with solvents of higher viscosity, because mass transfer is faster. Viscosity should be less than 0.5 centipoise, otherwise high pump pressures are required and mass transfer between solvent and stationary phase will be reduced.
3.1.10. Temperature
Temperature can have a profound effect on reversed phase chromatography, especially for low molecular weight solutes such as short peptides and oligonucleotides. The viscosity of the mobile phase used in reversed phase chromatography decreases with increasing column temperature. Since mass transport of solute between the mobile phase and the stationary phase is a diffusion-controlled process, decreasing solvent viscosity generally leads to more efficient mass transfer and, therefore, higher resolution. Increasing the temperature of a reversed phase column is particularly effective for low molecular weight solutes since they are suitably stable at the elevated temperatures9.
3.1.11. Other factors
Cost of fluorinated hydrocarbons precludes their use for all but the most critical work. Toxicity and flammability may pose safety and disposal problems. Chemical purity of solvent is also an important factor. Since large volumes of solvent are pumped through the column, trace impurities can easily concentrate in the column and can lead to false results or may harm the detector and column. Therefore, solvents of HPLC grade are recommended.
3.1.12. Detectors
A large numbers of detectors are used for RP-HPLC analysis. However, among these the five dominant detectors used in LC analysis are the electrical conductivity detector, the fluorescence detector, the refractive index detector, mass spectrometry detector and the UV detector (fixed and variable wavelength). These detectors are employed in over 95% of all LC analytical applications21-22
The detector selected should be chosen depending upon some characteristic property of the analyte like UV absorbance, fluorescence, conductance, oxidation, reduction, etc. Characteristics that are to be fulfilled by a detector to be used in HPLC determination are:
* High sensitivity, facilitating trace analysis
* Negligible baseline noise to facilitate lower detection
* Low drift and noise level
* Wide linear dynamic range (this simplifies quantitation)
* Low dead volume (minimal peak broadening)
* Cell design that eliminates remixing of the separated bands
* Insensitivity to changes in type of solvent, flow rate, and temperature
* Operational simplicity and reliability
* Tunability, so that detection can be optimized for different compounds
* Large linear dynamic range
* Non destructive to sample
4. CONCLUSION
Analytical methods development plays important roles in the discovery, development and manufacture of pharmaceuticals. RP-HPLC is probably the most universal, most sensitive analytical procedure and is unique in that it easily copes with multi-component mixtures. While developing the analytical methods for pharmaceuticals by RP-HPLC, must have good practical understanding of chromatographic separation to know how it varies with the sample and with varying experimental conditions in order to achieve optimum separation. To develop a HPLC method effectively, most of the effort should be spent in method development and optimization as this will improve the final method performance.
5. REFERENCES
1. Scott RPW. Principles and Practice of Chromatography. Chrom-Ed Book Series; 2003; 1-2.
2. Chatwal GR, Anand SK. Instrumental Methods of Chemical Analysis. 5th ed. Himalays Publishing House; 2004; 1.1-1.3, 2.566-2.2.575.
3. High performance liquid chromatography [Internet]. 2009 [Accessed 2009 Jan 20]. Available from: en. wikipedia.org/wiki/file:Agilent1200HPLC.jpg.html.
4. Beesley TE, Buglio B, Scott RPW. Quantitative Chromatographic Analysis. New York: Marcel Dekker; 2001; 378.
5. Patel RB. An Introduction to Analytical Method Development for Pharmaceutical Formulations [Internet]. 2008 [cited 2008 July 22]. Available from: pharmainfo
6. Willard HH, Dean AJ. Instrumental Methods of Analysis. 7th ed. New Delhi: CBS Publishers and distributors; 1986; 513-515, 580-604.
7. Connors AK. A Text Book of Pharmaceutical Analysis. 3rd ed. A Wiley Interscience publication; 2005; 373-400.
8. Ahuja S. High Pressure Liquid Chromatography. In: Ahuja S, Jespersen, editors.Comprehensive Analytical Chemistry. Elsevier; 2006.
9. Reversed Phase Chromatography: Principles and Methods. Amesham Biosciences; 6-8.
10. HPLC Separation Modes [Internet]. 2010 [Cited 2010 March 20]. Available from: waters.com/ aters/nav.htm?cid= 0049076&locale=en_US
11. How do I develop an HPLC method. Available from: www.sge.com
12. Snyder LR, Kirkland JJ, Glajch JL. Practical HPLC Method Development. 2nd ed. USA: John Wiley & Sons; 2001.
13. Sethi PD. HPLC Quantitative Analysis of Pharmaceutical Formulations. 1st ed. New Delhi: CBS Publishers & Distributors; 2001.
14. Lindholm J. Development and Validation of HPLC Methods for Analytical and Preparative Purposes. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology. 2004; 995.
15. Scott PWR. Liquid Chromatography for the Analyst. New York: Marcel Dekker Inc.; 1994. P. 1-10.
16. Stanley BJ, Foster CR, Guiochon G. On the Reproducibility of Column Performance in Liquid Chromatography and the Role of the Packing Density. J. Chrom. A. 1997; 761, 41-51.
17. Christain GD. Analytical Chemistry. 6th ed. USA: John Wiley & Sons Inc; 2001.
18. Kazakevich YV, Lobrutto R. HPLC for Pharmaceutical Scientists. 1st ed.USA: John Wiley & Sons Inc; 2007; 654-656.
19. Nledner W, Karsten M, Stelner F, Swart R. Automating Method Development with an HPLC System Optimized for Scouting of Columns, Eluents and Other Method Parameters. Pittcon presentation; 2008.
20. Kar A. Pharmaceutical Drug Analysis. 1st ed. New Delhi: Minerva Press; 2001; 565-592.
21. Beckett AH, Stenlake JB. Practical Pharmaceutical Chemistry. 1st ed. New Delhi: CBS Publishers and Distributors; 2002; 157-171.
22. Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. 8th ed. Singapore: Thomas Asia Pvt. Ltd; 2004; 973.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









