{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
1PRATYUSH KUMAR DAS*, 2SMRUTIPRAGNYA SAMAL, 3RATIKANTA SAHOO, 4PRASANT KUMAR SABAT
1,2,3Centre for Biotechnology, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India – 751003
4School of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India – 751003
*pratyushdas@soa.ac.in
ABSTRACT
Serratia marcescens, a gram negative bacillus is generally related to hospital acquired infections. Production of red pigment (Prodiogisin) by the bacterium is an important characteristic feature and has been reported to exhibit certain antimicrobial property. The resistance of the bacteria towards several classes of antibiotics makes it one of the most notorious pathogen. The work aimed to evaluate the resistance of Serratia marcescens (SPKD15) under various environmental stress conditions (temperature, pH, Salt concentration and UV). Effect of these stress conditions on the cell viability and production of prodiogisin was analysed. The strain was able to sustain up to temperature of 40°C, Salt (NaCl) concentration of 7%, pH up to 10 and could withstand UV radiations up to 2 minutes. However, the prodiogisin production was negatively affected and finally inhibited at all the conditions of environmental stress(Temperature = 35°C, pH = 4 and 9, Nacl concentration = 4% and UV exposure of 15 seconds). This indicates that prodiogisin may act as a protective mechanism for the bacterium under stress. Further, intracellular antimicrobial was obtained from the UV exposed culture and compared with the antimicrobial obtained from the normal culture. The antimicrobial obtained from UV exposed culture showed decreased antimicrobial effect with inhibition diameter ranging between 2 mm to 13 mm (± S.D) as compared to the antimicrobial obtained from the normal culture. An inhibition diameter ranging between 4 mm to 22 mm (± S.D) was obtained in case of the normal culture which may be attributed to the loss of pigmentation. The study highlights the resistance of the bacterium to various environmental stresses. Keeping the degree of pathogenicity of the bacterium in mind, eradication of the same is quite difficult and must be looked upon seriously.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2579
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 4 Received On: 22/02/2018; Accepted On: 07/03/2018; Published On: 01/04/2018 How to cite this article: Das PK, Samal S, Sahoo R, Sabat PK; Resistance of Serratia marcescens (SPKD15) to various environmental stress conditions: Effect on cell viability and prodiogisin production; PharmaTutor; 2018; 6(4); 18-26; http://dx.doi.org/10.29161/PT.v6.i4.2018.18 |
INTRODUCTION:
Serratia marcescens is a gram negative bacillus mostly responsible for hospital – acquired infection. Previously also known as Chromobacterium prodigiosum, the name Serratia marcescens was assigned in 1823 by Bizio (Hejazi and Falkiner, 1997). The most important characteristic feature of Serratia is production of red color pigment associated with the cell, called Prodigiosin. This pigment is generally not synthesized in strains isolated from infected adults (Singlton and Sainsbury, 2001; Ding and Williams, 1983). The pigment color varies from pink to dark or blood red depending on the age of colonies (Bunting, 1942). S.marcescens is mainly related to nosocomical infections, infections of urinary tract and was previously considered to be a non-pathogenic and saprophytic water organism. It was generally used as a biological marker because of its red colonies. Professor Scheurlen of the University of Strasbourg in 1896 concluded that the organism lead to more number of deaths as compared to many other pathogenic bacteria (Wheat et al., 1951). S.marcescens has been also reported to cause respiratory tract infection, meningitis, septicaemia and wound infections (Gouin et al., 1993) along with infective endocarditis (Mills and Drew, 1976). Yang et al., (2012) reported that S.marcescens also shows resistance towards a number of antimicrobial agents including β-lactams, Fluoroquinolones and Aminoglycosides.
The objective of the study was to analyze the resistance of Serratia marcescens (SPKD15), to various environmental stress conditions (Temperature, Salt concentration, pH and UV) with respect to cell viability and prodiogisin production. Further, the effect of UV on the production of intracellular antimicrobial by the strain was analyzed to establish a probable conclusion.
METHODS:
Isolation of Bacteria:
The desired bacterium was isolated from garden soil obtained from the premises of Srirama Chandra Bhanja Medical College and Hospital, Cuttack, Odisha by serial dilution technique, followed by the spread plate method. Further the colony morphology was studied and the desired colony (red colored, Smooth and glistening surface) was sub-cultured by streak plate method. The bacterial culture was then subjected to gram staining using HIMEDIA staining kit. The prepared slides were observed under 100X oil immersion objective to confirm the structure of the cell wall and the presence or absence of spores respectively.
Biochemical Identification:
Biochemical identification was carried out by API 20E test – a strip containing 20 different ready to use media for the purpose of biochemical identification of enterobacteriaceae species (Ingledew et al., 1980).
16S r RNA Sequencing:
In the process, first the bacterial chromosomal DNA was isolated by ROSE (Rapid One Step Extraction) method (Steiner et al., 1995) and quantified. Amplification of 16S rRNA gene was carried out using genomic DNA of Serratiamarcescens SPKD15 as the template along with universal primers – 8F (5'AGAGTTTGATCCTGGCTCAG3') and 1492R (5'GGTTACCTTGTTACGACTT3'). The PCR clone was sequenced by SCI Genom (P.) Ltd., India.
Comparison and Submission of Sequence:
The sequences received was subjected to Mega BLAST and compared with other available sequences in the National Centre for Biotechnology Information (NCBI)Genbank database. Before submission the sequences were checked and cleaned for “Chimeras” using online software – DECIPHER (Wright et al., 2012) and then submitted to NCBI.
Antimicrobial Susceptibility Test:
Antimicrobial susceptibility test on the isolated strain was carried out by using Kirby-Bauer’s disc diffusion method as per the guidelines of Clinical Laboratory Standard Institute (CLSI). The antibiotic discs taken for analysis are Imipenem (IC 10/10 µg), Piperacillin (PI 100µg), Amoxyclav (AMC 30µg) and Cefoxitin (CX 30µg) of HIMEDIA make. Based on the zone of inhibitions measured, the data was used to generate an antibiogram report by using ABIS online antibiogram analyser 1.0 software (ABIS ONLINE).
Effect of Stress conditions on growth of Serratia marcescens (SPKD15) and Prodigiosin production:
Effect of various stress conditions viz. temperature, salt concentration, pH and exposure to UV on the growth of Serratia marcescens SPKD15 and production of Prodigiosin(Red Pigment) was studied by performing colony count and calculating the percentage in relation to the control.
Effect of temperature:
The bacterial culture broth was spread on different petri plates containing nutrient agar medium and incubated under different temperature conditions (20⁰C - 45⁰C) for 24-48 hours. Results were noted thereafter (Noor et al., 2003).
Effect of salt concentration:
Similarly, bacterial culture spread on different nutrient agar plates containing NaCl concentration of 1%, 2%, 3%, 4%, 5%, 6% and 7% respectively were incubated for 24-48 hours, followed by observation (Aneja, 2003).
Effect of pH:
The bacterial culture was grown in various pH conditions ranging from pH 4 to pH 10 in nutrient hi-veg broth (HIMEDIA) for 24 hours at the optimum temperature of 25°C-27°C. The observation was noted after the incubation period (Aneja, 2003).
Effect of UV exposure:
100µl each of Serratia marcescens SPKD15 culture was spread on nutrient agar plates (in triplicates) and exposed to UV at 254 nm for a period of time ranging from 10 seconds to 3 minutes respectively and then incubated along with controls. Moreover, several streak plate (pure) culture of the strain was exposed to UV for a period of 5, 10, 20 and 30 minutes respectively. The UV exposed pure culture were again sub cultured on to freshly prepared nutrient agar plates (Aneja, 2003).
Effect of exposure to UV on production of intracellular antimicrobials by Serratia marcescens SPKD15:
The streak (pure culture) plate was exposed to UV by keeping the plate with its lid open at a distance of 15 cm away from the UV lamp inside a laminar airflow for duration of 30 minutes. After exposure to UV for the mentioned time, culture from the exposed plate was further sub-cultured in freshly prepared nutrient agar media. Microbial culture from the sub-cultured plate was taken by the help of an inoculation loop and was mixed with 50 ml of freshly prepared Luria Bertani (LB) broth and incubated for duration of 24 hours. After incubation, intracellular antimicrobial was extracted from the broth culture following the protocol established by Das et al. (Das et al., 2014). The same process was carried out to extract antimicrobial from the normal culture (control). The intracellular antimicrobial thus obtained from the bacteria after being exposed to UV, was used to study its inhibitory effect on various gram positive and gram negative microorganisms by agar well diffusion method. The antimicrobial obtained was tested against 4 bacterial cultures viz. Pseudomonas aeruginosa(ATCC 27853), Bacillus subtilis (MTCC121), Staphylococcou saureus (MTCC1144) andKlebssiella pnemoniae (MTCC432).The test was carried out in triplicates in order to avoid redundancy in results.The results obtained were compared with that exhibited by the controlto draw a meaningful conclusion.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSIONS
The present study reveals the resistance of Serratia marcescens SPKD15 to various environmental stress conditions. The isolated strain was found to be gram negative rod shaped bacterium (Fig.1) and was confirmed to the genus level by API 20E test (Table-1 and Fig.2) and other biochemical tests conducted. The bacterium isolated was confirmed to belong to the genus Serratia. The species was confirmed after 16S sequencing of the rRNA and the partial sequence (868 bp) was submitted to the NCBI genbank (accession no. KR422622). The strain was named as SPKD15.
Fig.1. Gram negative Serratiamarcescens SPKD15 as seen under 100X oil immersion objective.

Fig.2. The API 20E Test
Table.1. Results of API 20E Tests
|
Sl. No. |
Tests |
Results (+/-) |
|
1 |
β - galactosidase |
+ |
|
2 |
Arginine dihydrolase |
+ |
|
3 |
Lysine decarboxylase |
+ |
|
4 |
Ornithine decarboxylase |
+ |
|
5 |
Simmon’s citrate |
+ |
|
6 |
H2S Production |
- |
|
7 |
Urease |
+ |
|
8 |
Tryptophanase deaminase |
- |
|
9 |
Indole |
- |
|
10 |
VP |
- |
|
11 |
Gelatin hydrolase |
+ |
|
12 |
Glucose |
+ |
|
13 |
Mannitol |
+ |
|
14 |
Inositol |
+ |
|
15 |
Sorbitol |
+ |
|
16 |
Rhamnose |
- |
|
17 |
Saccharose |
+ |
|
18 |
Melobiose |
+ |
|
19 |
Amygladin |
+ |
|
20 |
Arabinose |
+ |
( + denotes positive result and - denotes negative result)
The antibiogram report (Fig.3) generated by the ABIS Online Antibiogram software indicated that the strain is susceptible to only one of the antibiotic whereas it is resistant to the other three. The strain SPKD15 was susceptible to Imipenem (10µg) belonging to the group Carbapenems, while resistance was observed towards antibiotics belonging to the group – Penicillins (Piperacillin 100µg), Beta-lactams (Amoxyclav 20+10µg) and Cephems (Cefoxitin 30µg).

Fig.3. Antibiogram report generated by ABIS Online Antibiogram Interpreter
Experiments carried out to study the effect of environmental stress factors (temperature, salt concentration, pH and exposure to UV) provided some interesting results. Serratia marcescens SPKD15 was found to be able to survive to an extent of 40°C, but the production of prodigiosin ceased beyond the temperature of 35°C. Although the bacteria lose its ability to produce colored pigment but still it can survive up to appreciably high temperatures. At 41°C almost 90-95 % of the bacterial cells did not survive, whereas 100% of the cells were able to produce prodigiosin at 28°C and approximately 80% of the cells produced the pigment at 30°C. Thereafter there was a sharp decline in the ability of cells to produce prodigiosin and approximately 88% of the colonies lose their ability to produce the pigment at 35°C. At a temperature of 37°C, all the bacterial colonies were devoid of the pigment (Fig.4).

Fig.4. Effect of temperature on viability and prodiogisin production of SPKD15
Similarly, the strain was also able to sustain high salt concentration up to 6% without much significant loss of growth (almost 58% of the cells survived), while the cell survival was limited up to 10-15 % at a salt concentrationof 7 percent. Pigment formation was unaltered at a salt concentration percentage of 1, while there was a decrease in pigmented cells up to 30 percent at concentration of 2% NaCl and almost 75 % reduction in prodigiosin was observed at 3% NaCl, followed by no formation of the pigment at NaCl concentration of 4 % (Fig.5).
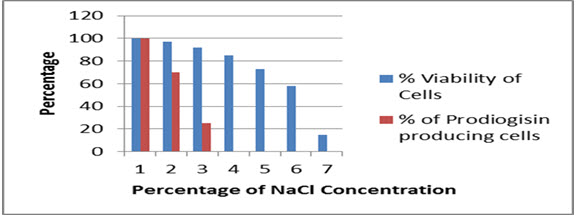
Fig.5. Effect of NaCl concentration on viability and prodiogisin production in SPKD15
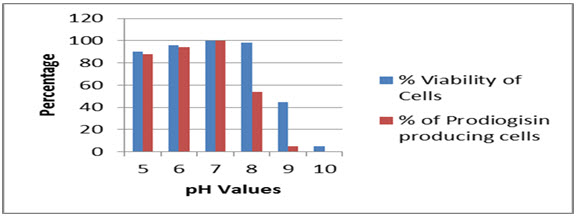
The strain SPKD15 was able to withstand a wide pH range from 5 to 9, whereas the cells were able to produce the pigment from pH 5 to 8. The optimum pH for production of prodiogisin was found to vary within 6-7 (Fig.6).
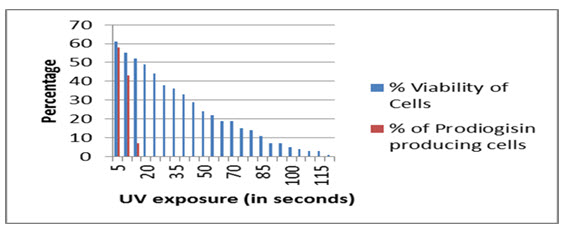
Serratia marcescens SPKD15 when exposed to 254 nm UV was able to survive the same up to maximum of 2 minutes duration. The pigment production capacity however was affected after exposure for 15 seconds and was completely ceased after 15 seconds of exposure (Fig.7).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Strain SPKD15 (streak plate culture) after being exposed to UV for 30 minutes, was subsequently sub-cultured and the sub-culture was used to extract antimicrobial. The antimicrobial thus obtained was tested against various test organisms and compared with the activity of antimicrobial obtained from the original strain SPKD15. The results of the tests are mentioned in Table.2.
Table-2. Comparison of activity of antimicrobials produced from normal and UV exposed strain

There was a notable difference observed among the activity of antimicrobial produced by the original strain and that produced from the UV exposed culture. Not only the antibacterial activity decreased but the UV exposed culture lost its pigment producing ability which can be physically ascertained by comparing the antimicrobial solutions obtained from both the culture. The antimicrobial solution extracted from the control culture had a rosy pink coloration whereas that obtained from the UV exposed culture had no color (Fig.8).

Fig.8. Antimicrobial obtained from UV exposed culture and normal strain.
The loss in coloration is possibly due to the degeneration of the prodiogisin pigment which may be due to alterations in the biochemical pathway leading to formation of the pigment. The positive aspect from this experiment was that the streak plate culture (strain SPKD15) after being exposed to UV for a long duration of 30 minutes was still able to grow on fresh nutrient media upon sub-culturing.
Environmental conditions are rarely stable and more often fluctuate between the optimum and adverse limits. Thus, the need to study the effect of various environmental parameters on a pathogenic microbe like Serratia marcescens arises. Such a study not only confirms the degree of resistance of the pathogenic bacteria, but also indicates towards possible evolution it can undergo over a period of time acquiring even more resistance. Several experiments on the impact of fluctuating environments on microbes such as pH (Hughes et al., 2007), temperature (Ketola et al., 2013), NaCl concentration (Rjazantseva et al., 1994) have been carried out earlier. The bacterium is able to grow at high temperatures, pH, salt concentration and can also resist UV for an extensive period. The bacterial strain survived up to 40°C and the optimum temperature for prodigiosin production was found to be 28°C. However the pigment formation ceased at 35°C. A similar result was also obtained by Andreeva et al. (Andreeva et al., 1999). The loss of pigmentation at higher temperatures may be due to the denaturation of heat sensitive enzymes involved in formation of the pigment. Similarly, the optimum pH for the production of prodiogisin was found to be at near about neutral which was confirmed by the intense coloration of the bacterial colony. However, further increase in alkalinity or acidity of the media lead to gradual loss of the pigments followed by loss of cell viability. This leads to the assumption that the pigment synthesis is somewhat responsible for imparting bacterial resistance towards fluctuations in pH. The isolated bacterial strain (SPKD15) could easily survive up to 6% NaCl concentration, very similar to the result obtained by Singh and Jha who demonstrated the growth of Serratia marcescens (CDP-13) at a higher NaCl concentration and application of the same to enhance the growth of wheat plant under salinity stress conditions (Singh and Jha, 2016). However, the cells of the bacterial strain (SPKD15) almost lost their ability to form pigments at a pH of 3 – 4, which is in quite contrast to the results obtained by Rjazantseva et al. showing late synthesis of pigments at low concentration of NaCl and higher accumulation of the prodiogisin pigment up to 5% w/v NaCl concentration (Rjazantseva et al., 1994). The probable reason for such conflicting results may be attributed to the difference in strain. It may be concluded that the activity of Serratia marcescens towards high salt concentration may vary from strain to strain. Strain SPKD15 was able to survive an extensive period of UV exposure of up to 120 seconds; however the production of prodiogisin was highly affected after 15 seconds of exposure. According to some researchers the higher resistance of S.marcescens to radiations is mainly due to the presence of red colored pigment called prodiogisin, which is somewhat structurally similar to that of melanin (Bartlett et al., 1970; Webb et al., 1971). However, on the other hand it has been observed that there is no significant effect of resistance between pigmented and non-pigmented cultures of S.marcescens towards UV as confirmed by Zion et al (Zion et al., 2006). The survival ability of S.marcescens under prolonged exposure to UV may be attributed to the presence of efficient DNA repair mechanism in the bacteria. At certain point the damage due to UV radiation may reach a certain critical level beyond which the DNA repair mechanism may not be able to work properly, thus leading to loss of cell viability. The mutations to the DNA may also impact the production of prodiogisin adversely by blocking the pigment production pathway.The decreased activity of the antimicrobial produced from the UV exposed strain may be attributed to the possible mutations to specific genes or blockage of pathway that leads to production of prodiogisin. The pigment prodiogisin is well known to have certain antimicrobial activity and hence, its degeneration or lack of synthesis may reduce the intracellular antimicrobial property. The bacteria although lacks prodiogisin after exposure to UV and sub-culturing, but still exhibits certain antimicrobial activity. This reflects that prodiogisin is not the only factor behind the antimicrobial property but some other factors must be contributing to the same cause.
All the experiments carried out signify the tolerance of Serratia marcescens (SPKD15). It is not only resistant to certain antibiotics as studied earlier (Cooksey et al., 1975; Sleigh, 1983) but also is highly resistant to drastic environmental stress.The survival of the bacterium may be due to the prodiogisinpigment, which may act as a shield and protect the bacteria from harsh environmental conditions. The logic applied behind such interpretation is that the bacterium loses the pigment forming ability under harsh environmental conditions at first, followed by the loss of viability. Hence, the pigment prodiogisin is thought to act as the first line of defense for the bacterium under drastic environmental stress. Besides, the bacterium is assumed to pose efficient DNA repair mechanism which also is an added benefit for its survival. The resistance of this particular species to such stress conditions makes it even more notorious as a pathogenic organism.
CONCLUSION
Serratia marcescens already known for its pathogenicity is highly resistant to large classes of antibiotics. The study reveals the resistance of the bacteria towards various environmental stress conditions. The bacteria are able to survive at high levels of temperature, salt concentration, pH and extended duration of UV exposure. The high level of tolerance exhibited by the isolated strain makes it difficult for proper treatment of infections caused by it. This demands new insights for further research in the field to make out possible ways for efficient eradication of the pathogenic bacteria.
REFERENCES
1. Andreeva I.N., Ogorodnikova T.I.; The effect of the cultivation conditions on the growth and pigmentation of Serratia marcescens; ZhMikrobiol Epidemiol Immunobiol.; 1999; Vol. May-Jun (3); 16-20.
2. Aneja K.R.; Experiments in Microbiology, Plant Pathology and Biotechnology. 4thedition. New Delhi: New Age International; 2003. ISBN: 81-224-1494-X
3. Bartlett W.T., O’Donovan G.A., Neff R.D.; Effect of gammaradiation on Serratia marcescens. Studies on the radiosensitivity ofprodigiosin production; Radiation Research; 1970; Vol. 43; 196-203.
4. Bunting M.I; Factors Affecting the Distribution of Color Variants in Ageing Broth Cultures of Serratia marcescens #274; J. Bacteriol.; 1942; Vol. 43; 593-606.
5. Cooksey R.C., Bannister E.R., Farrar Jr. W.E.; Antibiotic Resistance Patterns of Clinical Isolates of Serratia marcescens; Antimicrob Agents Chemother; 1975; Vol. 7 No. 4; 396-399.
6. Das P.K., Das S., Sahoo D., Dalei J., Rao V.M., Nayak S., Palo S.; Comparative Evaluation of Purification Methods for Production of Polypeptide Antibiotics – “Polymyxin B” and “Cerexin A” from Bacillus Species; PharmaTutor; 2014; Vol. 2 No. 8; 188-200.
7. Ding M.J. and Williams R.P.; Biosynthesis of prodigiosin by white strains of Serratia marcescens isolated from patients; J. Clin. Microbiol.; 1983; Vol 17; 476-80.
8. Gouin F., Papazian L., Martin C. et al.; A non-comparative study of the efficacy and tolerance of cefepime in combination with amikacin in the treatment of severe infections in patients in intensive care; J AntimicrobChemother; 1993; Vol. 32 Suppl B; 205-214.
9. Hejazi A. and Falkiner F.R.; Serratia marcescens; J. Med. Microbiol.; 1997; Vol 46; 903-912.
10. http://www.tgw1916.net/bacteria_logare_desktop.html
11. Hughes B.S., Cullum A.J., Bennett A.F.; An experimental evolutionary study on adaptation to temporally fluctuating pH in Escherichia coli; Physiol. Biochem. Zool.; 2007; Vol. 80 ; 406–421.
12. Ingledew W.M., Sivaswamy G., Burton J.D.; The API 20E microtube system for rapid identification of gram negative brewery bacteria; J. Inst. Brew.; 1980; Vol. 86 (July-August); 165-168.
13. Ketola T.L.,Mikonranta J., Zhang K., Saarinen A.M., Ormala et al.; Fluctuating temperature leads to evolution of thermal generalism and preadaptation to novel environments; Evolution; 2013; Vol. 67 ; 2936–2944.
14. Mills J., Drew D.; Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse; Ann Intern Med.; 1976; Vol 84; 29-35.
15. Noor R., Islam Z., Munshi S.K., Rahman F.; Influence of Temperature on Escherichia coli Growth in Different Culture Media; J Pure ApplMicrobio.; 2003; Vol. 7 No. 2; 899-904.
16. Rjazantseva I.N., Andreeva I.N., Ogorodnikova T.I.; Effect of various growth conditions on pigmentation of Serratia marcescens. Microbios.; 1994; Vol. 79; 155- 161.
17. Sleigh J.D.; Antibiotic resistance in Serratia marcescens; Br Med J (Clin Res Ed); 1983; 3 (6406): 1651-1652.
18. Singh R.P., Jha P.N.; The Multifarious PGPR Serratia marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticumaestivum L.); PLoS ONE; 2016; Vol. 11 No. 6; e0155026. doi:10.1371/journal.pone.0155026
19. Singlton P. and Sainsbury D.; Dictionare of Microbiology and Molecular Biology. 3rd Edn. Johan Willy and Sons Ltd.
20. Steiner J.J., Poklemba C.J., Fjellstrom R.G. and Elliott L.F.; A rapid one tube genomic DNA extraction process for PCR and RAPD analyses; Nucleic Acids Res.; 1995; Vol. 23; 2569–2570.
21. Webb P.S., Neff R.D., O’Donovan G.A.; Effect of gammaradiation on Serratia marcescens. Comparison of the radiosensitivity of pigmented and nonpigmented cells; Radiation Research; 1971; Vol. 48; 40-52.
22. Wheat R.P., Zuckerman A., Rank L.A.; Infection due to Chromobacteria: report of eleven cases; Arch Intern Med; 1951; Vol. 88; 461-466.
23. Wright E.S., Yilmaz L.S., Noguera D.R.; DECIPHER, A Search-Based Approach to Chimera Identification for 16S rRNA Sequences; Applied and Environmental Microbiology; 2012; Doi:10.1128/AEM.06516-11.
24. Yang H., Cheng J., Hu L., Zhu Y., Li J.; Mechanisms of antimicrobial resistance in Serratia marcescens; Afr. J. Microbiol. Res.; 2012; Vol. 6 No. 21; 4427-4437. Doi: 10.5897/AJMR11.1545.
25. Zion M., Guy D., Yarom R., Slesak M.; UV radiation damage and bacterial DNA repair systems repair; Journal of Biological Education; 2006; Vol. 41 No. 1; 30-33. Doi: 10.1080/00219266.2006.9656054.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









