About Authors: Ms.Komal R. Nikam (B.Pharmacy), Mr.Mahendra G. Pawar (B.Pharmacy), Mr. Kishor S. Salunkhe (M.Pharm,PhD), Mr.Devidas G.Bachhav(M.Pharm)
Amrutvahini college of pharmacy,Sangamner.(Pune University)
Abstract:
The demand for novel drug delivery technologies is ever increasing. These drug delivery technologies includes oral, transdermal, inhalation and parenteral. The main goal for the delivery of any drug therapy is oral administration with once or twice daily dosing. However, there are large numbers of therapies, particularly protein-based, gene-based vaccine-based that cannot be delivered by this route for example insulin, growth hormones and other similar biologicals.
The pitfalls of needle-based injections are well known. Psychological resistances to self-injection or needle-phobia have been documented across large demographic groups, such as diabetics. The result of this phobia is that many outpatient injectables are dosed sub-optimally. Furthermore, awareness of serious problems has caused physicians and their patients to either delay therapy initiation or seek out less-invasive alternatives, even at some cost to clinical effectiveness.
For some, especially those suffering from chronic diseases requiring injectable products two or three times a day, this process is an ongoing reality of daily life for example diabetics-accepted, but always with the hope that something new will replace the ritual of needle insertion. To overcome the problems related to needle based injections, there is one technology that has received considerable attention during the past few years and that offers all of the sought after benefits is—Needle Free Injection Technology (NFIT).
[adsense:336x280:8701650588]
INTRODUCTION:
Diabetes mellitus is chronic, progressive, systemic disease characterized by the dysfunction of metabolism of fat, carbohydrates, protein, insulin, function and structure of blood vessels and nerves. It is projected to become of the world’s main disablers and killers with in the next 25 years. Currently there are over 150 million diabetics worldwide and this number is likely to increase to 300 million or more by the year 2025 due to increase in sedentary lifestyle, consumption of energy rich diet and obesity. Diabetes mellitus represents a group of diseases of heterogeneous etiology, characterized by chronic hyperglycemia and other metabolic abnormalities. The etiological classification of diabetes includes type I, type II, those due to specific mechanisms or diseases and gestational diabetes. Diabetes mellitus type I is characterized by destructive lesions of pancreatic β cells by an auto-immune mechanism. Type II diabetes is characterized by a combination of decreased insulin secretion and sensitivity. Attempts to attain strict glucose control when managing diabetes have traditionally utilized daily subcutaneous injections of human insulin. This strategy has offered improvements in glycemic control but is unable to replicate fully the normal, diurnal plasma profile of endogenous insulin[1]. The development of novel noninvasive routes of insulin administration promises to further improve diabetes management. Many barriers to initiate insulin therapy include need for frequent insulin injection, fears that insulin injections will be painful and difficult to administer, concerns about hypoglycemia and weight gain.
Traditional insulin drug delivery system:
Insulin therapy via subcutaneous or other parenteral route in diabetic patient is preferred but on continuous administration there may be chance of peripheral hyperinsulinemia, formation of thrombus, inflammation & irritation at the site. Also patient suffering from needle phobia hesitate to take it.
Historical background of painless insulin drug delivery system:
This technology was first described in 19th century in France, when the company H Galante-manufactured an ‘Apparatus for Aqua puncture’. Since then, the demand had increases considerably. It was first commercialized in the US in 1960’s. Biojet had summed up the reason for it in their brochure stating” Patients hate needles, healthcare professionals fear accidental needle stick injuries ,drug companies are looking for new and innovative ways of delivering their products”. [2]
NEEDLE FREE TECHNOLOGY: [1,,3,4]
The reason this topic fits is that the diabetes market and particularly insulin development is, by its nature, a hotbed for new ideas in drug delivery. It is difficult to think of one other therapeutic area, let alone another single disease, where so many factors align to drive the development of novel delivery systems.
Needle free insulin injection provides following advantages:
i. Improve concordance with insulin regiment.
ii. Improve the patient health/well-being.
iii. Eliminates the need for sharp disposal and avoids needle stick injuries.
IV .Emotional benefits of using a needle free devices.
v. No sharp to dispose off.
vi. Fast injection, insulin is delivered in less than 0.3 seconds, regardless of dose.
vii. No additional pressure required to delivered large doses.
Additional benefits: Very fast injection compared with conventional needle and no needle disposal issue. Organisations such as W.H.O. and C.D.C. (center for disease control) support the development of needle free insulin drug delivery.
DEVICES USED IN PAINLESS INSULIN DRUG DELIVERY SYSTEM:
1. Jet injectors: [2, 10, 11]
A jet injector is a type of medical injecting syringe that uses a high-pressure narrow jet of the injection liquid instead of a hypodermic needle to penetrate the epidermis. It is powered by compressed air or gas, either by a pressure hose from a large cylinder, or from a built-in gas cartridge or small cylinder. Some are multi-shot, and some are one-shot. They are used by diabetics to inject insulin as an alternative to needle syringes, though they are still not very common.
Types of jet injector:
1.1 Jet Injectors Gun:
The Jet Injector Gun (Fig: 1) and the Ped-O-Jet is air-powered medical injector devices designed to administer vaccinations in an extremely efficient manner. Invented by Aaron Ismach. The Jet Injector is powered by electricity, while the Ped-O-Jet version is powered by a foot pump and does not require electricity to administer the vaccines. These devices have various specialized nozzles for different medication densities and also permitted the efficient inoculation of animal populations as well. Care must be taken around high pressure sprays of this kind to avoid such injuries.

Fig. no. (1): Jet Injector

Fig. no. (2): “Elephant Ear” model of Biojector
Device Preferred by children:
In numerous surveys conducted by Bioject, patients consistently prefer the Biojector 2000 over needle-and-syringe. Most patients fear the pain and discomfort of needle-based injections. However, Bioject does not claim that its systems are painless. Some medications and vaccines, for instance, can cause burning and stinging sensations because of their formulations, which are independent of the injection method. For highly needle-phobic patients, such as small children, the biojector has optional "Elephant Ears," (Fig: 2) which can help reduce anxiety.
1.2 Hypodermal jet injectors without needle "JET 2000":
This injector offers the users a valuable alternative to the traditional syringe and it is a painless mean for the administration of medicines. While the traditional syringe penetrates the cutaneous barrier by means of a needle, the injectors administers the injectable substance at a sub-cutaneous level by the pressure generated through a spring system controlled by a switch. Also the cutaneous absorption is faster than the one given by a traditional syringe thanks to a major depth of injection and to a larger cutaneous area concerned. In this way, it is possible to avoid any kind of trauma and the liquid substance is rapidly absorbed by the sub-cutaneous vascular system.
1.3 Low Workload Jet Injector for Routine Immunizations:
Routine immunization clinics around the world deliver vaccines to children using needles and syringes, a simple, effective, and inexpensive method for parenteral delivery of drugs and vaccines. However, because of improper sterilization and possible reuse of the needle and syringe, the danger of transmission of blood-borne disease is very high, especially in resource-poor settings.
In an attempt to eliminate the problems associated with needles and syringes, multi-use nozzle jet injectors, were once used for immunizations until they were found to transmit blood borne pathogens between patients. Their use was stopped and the world was left with no alternative to needles for immunization.
1.4 Multi-Channel Jet Injector for Simultaneous Vaccine Delivery:
New childhood vaccines are being developed rapidly, but children, parents, and providers are less willing to accept the increased numbers of separate injections required at a single doctor visit. Missed immunizations can result in increased disease occurrence among unprotected children. Combination vaccines are an alternative, but potential chemical incompatibilities require years of expensive research and regulatory approval to ensure that vaccine efficacy and safety are not compromised. The objective of this project was to determine the scientific, technical and commercial merit and feasibility of a multi-channel jet injector to deliver multiple vaccines simultaneously.
1.5 Biojector 2000:
The Biojector 2000 is an innovative, versatile needle-free injection system that has been used to deliver millions of injections in a wide range of healthcare settings. The Biojector has many unique features, including the ability to deliver both intramuscular and subcutaneous injections up to 1 ml in volume. Since its introduction in 1993, the Biojector has set a new standard for the comfort, safety, and convenience of injection equipment.
The Biojector has FDA clearance for delivering subcutaneous or intramuscular injections of liquid medication, including vaccines and other injected medications.
Because there is no needle, the Biojector provides healthcare workers with an unparalleled level of protection against accidental needlestick injuries. In high-risk situations, such as delivering injections to patients known to be infected with HIV or Hepatitis, the Biojector is an ideal injection system.
The Biojector works by the same principle as all of Bioject's needle-free injection systems: by forcing liquid medication through a tiny orifice that is held against the skin. This creates a very fine, high-pressure stream of medication that penetrates the skin, depositing medication in the tissue beneath.
The system has three components: a durable injection device, a disposable needle-free syringe, and a CO2 cartridge.
2. Inhalable insulin: [2, 11, 12]
Inhalable insulin (Fig: 3) was available from September 2006 to October 2007 in the United States as a new method of delivering insulin, a drug used in the treatment of diabetes to the body. After the withdrawal of the only inhalable formulation, all currently available insulin formulations are administered by subcutaneous or intravenous injection.
The first such product to be marketed was Exubera, a powdered form of recombinant human insulin, delivered through an inhaler into the lungs where it is absorbed. Once it has been absorbed, it begins working within the body over the next few hours. Diabetics still need to take longer acting basal insulin by injection.
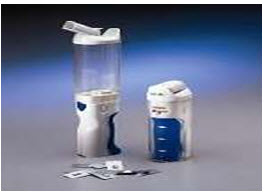
Fig. no. (3): Inhalers Used In Insulin Therapy
3. Insulin spray: [2, 11, 13]
The buccal route is another promising alternative for insulin delivery. With the buccal area having an abundant blood supply, it offers some advantages such as a means to deliver the acid labile insulin, and elimination of insulin destruction by first pass metabolism. The buccal spray (Fig:4)formulation being developed by Generex Biotechnology, based in Toronto, delivers insulin to the buccal cavity as a fine spray using company's 'rapidmist' device. The company’s leading product is oralin.The patient does not inhale with the buccal spray device; instead, the drug is sprayed onto the buccal mucosa. The high-speed spray allows the drug to be rapidly absorbed into the bloodstream. The deposition of the drug onto the buccal mucosa also allows the developers to bypass earlier concerns about any risks to lung tissue that have been raised regarding investigative inhaled insulin formulation.

Fig. no.(4): Insulin Spray
4. Insulin Pen: [2, 14, 15]
An insulin pen (Fig: 5) is an insulin injection system for the treatment of diabetes. A pen is comprised of disposable of pensneedles, a vial of insulin, and a "pen."
Types:
A number of companies make insulin pens including Novo Nordisk, Aventis and Eli Lilly. These companies produce pens for most of their insulins, including NovoLog/NovoRapid, Humalog, and Lantus.
There are two pen systems:
1. A replaceable cartridge pen reuses the pen portion. When the insulin is empty, the vial is replaced by inserting a new one.
2. A prefilled pen is entirely disposable. When the insulin is gone, the entire unit is discarded.
Use:
One significant advantage of pens is their ease of use. To use a pen- screw on a new needle. If necessary, prime the pen to remove any air from the needle .Turn the knob on the end of the pen (or "dial") to the number of units needed. Insert the needle into the skin. Press the button on the end of the pen to deliver the dose .Count to five & remove.
Advantages:
Insulin pens have a number of advantages:
i. More convenient and easier to transport than traditional vial and syringe.
ii. Repeatedly more accurate dosages.
iii. Easier to use for those with visual or fine motor skills impairments.
iv. Less injection pain (as polished and coated needles are not dulled by insertion into a vial of insulin before a second insertion into the skin)
Disadvantages:
Unlike the traditional syringe, pens are usually restricted to full or half unit dosing. You are also not able to mix two different insulins in the same pen. In addition, insurance coverage for insulin pens in the United States may vary widely.
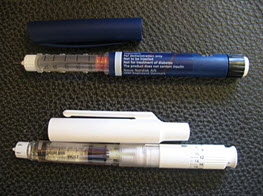
Fig. no. (5): Insulin pen
5. Insulin micropump: [2, 11, 16]
Flamel micro pumps technologies a controlled release system which permits delayed and extended delivery, of small molecule drugs. It is suitable in particularly narrow window of absorption from the upper part of the small intestine.
Description:
Flamel micro pump technology (Fig: 6) consists of a multiple per capsule or tablet containing micro particles per capsule or tablet. The 200-500 mm diameter size microform in the stomach and pass into the small intestine, where each micro particle, operation delivery system release the drug by osmotic pump at a adjustable rate (micro pump 1st or delayed for micro pump 2nd) and over and extended period of transit of time.
The design of micro pump micro particle allows extended transit time enough plasma mean resident time extended up to 24 hrs, which is spatially suited drug known to be absorbed only in small intestine the micro particle design can be adopted to be each drug specifically modifying the coating thickness and composition include the expedients (encapsulation) reduce toxicity and or reduces C max or peak drug concentration in the plasma (an improve patients regimen)
Setting up of insulin pump:

Fig. no. (6): Micropump
In order to use an insulin pump (Fig: 7), the reservoir must first be filled with insulin. Some pumps are designed to be used with prefilled cartridges that are replaced when empty. Most, however, must be filled with insulin of the user's choice (usually Novolog, Humalog, or Apidra). Setting up includes:
i. Opening a new (sterile) empty pump reservoir of the manufacturer's design;
ii. Withdrawing the plunger;
iii. Inserting the needle into a vial of insulin;
iv. Injecting the air from the reservoir into the vial to prevent a vacuum forming in the vial as insulin is withdrawn;
v. Drawing insulin into the reservoir with the plunger, and then removing the needle;
vi. Squirting out any air bubbles from the reservoir, and then removing the plunger;
vii. Attaching the reservoir to the infusion set tubing;
viii. Installing the assembly into the pump and priming the tubing (pushing insulin and any air bubbles through the tubing) - it is essential that this is done with the pump disconnected from the body to prevent accidental insulin delivery;
ix. Attaching to the infusion "site" (and priming the cannula if a new set has been inserted).
Dosing:
An insulin pump allows the replacement of slow-acting insulin for basal needs with a continuous infusion of rapid-acting insulin.
The insulin pump delivers a single type of fast-acting insulin in two ways:
i. Bolus dose that is pumped to cover food eaten or to correct a high blood glucose level.
ii. Basal dose that is pumped continuously at an adjustable basal rate to deliver insulin needed between meals and at night.
Future developments:
When insulin pump technology is combined with a continuous blood glucose monitoring system, the technology seems promising for real-time control of the blood sugar level. Currently there are no mature algorithms to automatically control the insulin delivery based on feedback of the blood glucose level. When the loop is closed, the system may function as an artificial pancreas.

Fig. no. (7): Insulin pump
6. Insulin port: [18, 19]
An insulin port (Fig: 8) functions as a medication delivery channel directly into the subcutaneous tissue. When applying the injection port, an insertion needle guides a soft cannula (a small, flexible tube) under the skin. Once applied, the insertion needle is removed and only the soft cannula remains below the skin, acting as the gateway into the subcutaneous tissue.
To inject through insulin port the needle of a syringe or insulin pen is used. The needle remains above the surface of the skin, while the medication is immediately delivered through the soft cannula and into the subcutaneous tissue.

Fig .no.(8): Insulin Port.
7. Transdermal patches: [20, 21, 22]
Ozin and Landskron announced recently that they had created an unusual material using manmade molecules called dendrimers. It can store drugs and when spread on the skin as a film, allow them to dissipate into a patient’s bloodstream like a new type of patch. The problem with current drug delivery systems is that it is either injected in such a manner that acquires too high concentration to ensure that it stays in the system but can be toxic, or it is injected too little into a person such that it is not effective. The new material, Periodic Mesophorus Dendrisillicus (PMD) would let drugs seep through a person’s skin in just the right amount and stay at that level.
The Altea Therapeutics Passport System was the first product in development shown in US FDA clinical trials to provide a non-invasive, controllable and efficient way to deliver insulin via a patch on the skin. The Passport System enables fast, controlled drug delivery without the pain of an injection or the possible complications associated with inhaled medications. It also avoids the first-pass gastro-intestinal and liver metabolism that occurs often after oral administration. It creates and effective, economical and patient-friendly delivery of insulin.
The insulin transdermal patch (Fig: 9) maintains constant basal levels while avoiding skin depots of insulin common with subcutaneous injections. As a safety feature, if a patient begins to experience the hypoglycemia associated with an inadvertent overdose of insulin, they may simply remove the insulin transdermal patch, thus immediately ending the influx of insulin.

Fig. no.(9): Transdermal patches
8. Insulin Nanopump: [23]
Based on the Nanopump technology, the Insulin Nanopump (Fig: 10) offers unmet improvement in diabetes therapy. It was designed with the help and assistance of diabetic patients, nurses and endocrinologists with the objective of bringing a significant contribution to patient’s quality of life. Conceived as a patch pump without tubing, it gives more flexibility and freedom to the patient. Its extremely small size and weight allows wearing it completely hidden under the clothes, its ultra-precision permits an accurate delivery of insulin even at very low delivery rates and independently of external conditions, its built-in functional monitoring guarantees perfect safety during its use and its general conception makes it more affordable for patients.

Fig. no.(10):Insulin Nanopump
9. Insulin Pills: [25]
To adequately control postprandial glycemia, several daily injections of insulin are necessary. However, insulin therapy via subcutaneous or other parenteral route is known to result in peripheral hyperinsulinemia. In addition to the risk of hypoglycemia, some studies have suggested that peripheral hyperinsulinemia may be associated with coronary artery disease, hypertension, dyslipidemia and weight gain[16]. There is strong evidence suggesting that an oral insulin product would provide insulin in a more physiological manner, with a resultant decrease in peripheral insulin concentration and that it would more adequately insulinize the liver[17, 18]. Azopolymer coated pellets to deliver insulin to the colon region were studied earlier. The azopolymer protects the entrapped therapeutic agent till the pellets reach the colon. As only the bacteria inhabiting the colon secrete enzymes that can breakdown the azopolymer, insulin release will be initiated once the pellets reach the large intestine[19]. Microencapsulation of insulin in polymeric microspheres coated with pH responsive polymers such as alginate is also known. Alginate coating protects the spheres in the acidic pH of the stomach but dissolves in the intestine where the pH increases to above 7 and liberates the entrapped insulin[20] .Recently several biotech companies have been conducting pilot trials in the effort to develop an insulin pill as a potential alternative to injected or pumped insulin. The attempt requires the development of novel delivery technology. For example, Nobex Corporation has developed hexyl-insulin monoconjugate 2 (HIM- 2) in which single amphiphilic oligimer is covalently linked to the free amino group on the Lys-β 29 residues of recombinant human insulin via an amide bond. This alters the physical- chemical characteristics, leading to enhanced stability and resistance to intestinal degradation of ingested insulin [21]. Oral HIM-2 is safe [22] and reproduces the physiological pathway of insulin secreted by pancreas [23]. Also Depomed, Inc. is developing oral medications using its Gastric Retention (GR) system, an advanced polymer-based, oral drug delivery formulation. Initially small enough to be easily swallowed by the patients, the pill swells following its ingestion. Simultaneously, the system begins a period of extended drug release. This sustained delivery could some day lead to an insulin pill (Fig: 11) that provides steady release into the bloodstream, minimizing the number of doses required per day.
In trials in Type I diabetic patients, the insulin pill helped control glucose levels as well or better as taking injections of short-acting insulin before meals.

Fig. no. (11): Insulin pill.
10. Insulin capsule: [26, 27]
Chemists have developed polymeric capsules (Fig:12) to protect insulin from destructive effect of digestive juices. Researchers of the Chemical Faculty, Lomonosov Moscow State University have found the way to protect insulin from the destructive effects of the digestive juices and to preserve the ability to perform its function.
These polymeric capsules are stable and remain intact in acid medium and they gradually excrete insulin in a neutral medium. The two polymers used are positive protamin and negative dextransulphate. They form layers in series one upon the other according to the plus towards minus principle and make a multi-layer covering around the insulin filling, which makes up to 85% of the entire microparticle.
Insulin covered by protective capsule is stable in acidic medium of pH from 1.7 to 5 units. When pH increases to a level above 5 units, insulin gets released. Further pH increase of up to 8 units results in accelerated protein release rate. Such behaviour of particles occurs due to the fact that at pH higher than 5.5 units of insulin acquires negative charge and its bond with the negatively charged polymer of the first layer dextransulphate gets destroyed. Such pH-dependence of protective polymeric capsules provides fundamental capability to create insulin in pills.

Fig. no.(12):Insulin Capsule
11. Insulin Tablets:
The great problem of taking insulin orally was reported about overcome. The problem has been to ingest this hormone. When swallowed stomach acids and enzymes destroy it, stomach fats
render any residues ineffective. Therefore victims of diabetes must inject insulin into their veins. Physiologist John Raymond Murlin of the university of Rochester announced that he and assistants have just perfected a compound of insulin and hexylresorcinol which may be swallowed as a tablet (Fig: 13). It is effective because the hexylresorcinol neutralizes pepsin and acid, and emulsifies fat. Thus there remains nothing to impede insulin’s absorption by the diabetic’s sugar-laden body.

Fig. no.(13):Insulin Tablets
12. Islet cell transplant: [28, 29,30]
In contrast to conventional insulin treatment, islet transplantation (Fig: 14) is far superior for achieving a constant normoglycaemic state and avoiding hypoglycemic episodes. Insulin-producing beta cells are taken from a donor's pancreas and transferred into a person with diabetes. Once transplanted, the donor islets begin to make and release insulin, actively regulating the level of glucose in the blood.
Successful transplantation can provide the following benefits:
i. It can eliminate the need for frequent blood glucose measurements and the need for daily insulin injections. Although only a few are free of insulin injections a year after transplantation. ii. It can provide more flexibility with meal planning.
iii. It can help protect against the serious long-term complications of diabetes, including heart disease, kidney disease, stroke and nerve and eye damage.
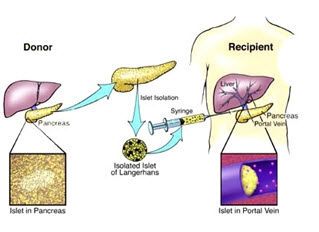
Fig. no.(14):Islet cell transplant
13. Insulin analogues: [31, 32]
In the early 1980’s, human insulin produced by recombinant-DNA technology became available for the treatment of diabetes. Subsequently, protein engineering has been used to produce variant forms of insulin, known as insulin analogues (Fig:15) , with modified amino-acid sequences and improved pharmacokinetic properties.
Rapid acting analogues :
Insulin lispro and insulin aspart are rapid-acting analogues that have reduced self-association as a result of protein engineering. In insulin lispro, a lysine-proline (Lys-Pro) sequence at the end of the insulin-B chain is reversed, which creates steric hindrance and a reduced ability to self-associate. Insulin aspart incorporates an amino-acid change (Pro B28 to aspartic acid (Asp)) that also creates charge repulsion and steric hindrance due to a local conformational change at the carboxyl terminus of the B chain. Both are absorbed more rapidly than regular insulin and reduce post-prandial glucose excursions more efficiently. Because of their short-lived action, adjustments in basal insulin levels are required to achieve improvements in overall glycemic control.
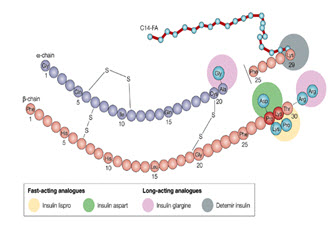
Fig. no.(15):Insulin Analogues
Long-acting analogues:
Insulin glargine is long-acting insulin that contains two extra arginine molecules at the end of the B chain (Arg B31 and Arg B32) to alter the isoelectric point. A glycine substitution at A21 (A chain) was made to stabilize the molecule. After subcutaneous injection, insulin levels rise slowly to a plateau within 6–8 hours and remain essentially unchanged for up to 24 hours, suitable for once-daily administration. Acylation of the -amino group of Lys B29, as in insulin detemir (NB29–myristoyl des (B30) human insulin), promotes reversible binding of insulin to albumin, thereby delaying its absorption from the subcutaneous tissue and transport across the capillary endothelium of skeletal muscle. This form of insulin is under development. C14-FA, myristoylic acid.
14. Insulin complement: [31]
One new drug Symylin is ready to be launched by Amylin Pharma, San Diego. Symylin is a synthetic vergion of human hormone Amylin, which moderates the glucose lowering effect of insulin. It is design to complement insulin action and has been shown to reduce blood glucose without causing an increase in hypoglycemic episodes. It could provide a potential adjunct to insulin therapy in both type I and type II diabetes.
15. Vaccine against diabetes:
A world-leading medical trial conducted in Melbourne suggests that the onset of type I diabetes could be prevented in many at-risk people by a new nasal insulin vaccine. The phase II trial at The Royal Melbourne Hospital in children at high risk of developing type I diabetes. Of the 38 children in the trial, 12 who started with very little or no insulin-producing function went on to develop diabetes within one to two years. However, of the other 26, all of whom began the trial with some of their own insulin-producing function, none developed diabetes after three years. The results from the trial are very encouraging. First, the use of nasal insulin has been established as being safe. Second, it was found that nasal insulin issued protective instructions to the immune system in humans.
16. Stem cell technology:
A better treatment for type I diabetes is just one of the hopes for stem cell therapy. Scientifically more promising ethically but more problematic are stem cells derived from human embryos. Most of the stem cells now being used in research were originally sourced either from leftover IVF embryos or from aborted foetuses. In the case of IVF procedures, a five-day-old embryo called a blastocyst is implanted into a woman's uterus, and hopefully, nine months later, a baby is born. Any extra embryos are usually kept in case of miscarriage, or for a future pregnancy, but they are not always required. To create human embryonic stem cells for research, some of the blastocyst's cells are isolated, harvested and allowed to grow in a separate dish. To turn them into long-lasting stem cell lines, the cells are fed special growth factors. The embryo is destroyed in the process.
FUTURE PROSPECTIVES FOR PAINLESS INSULIN DRUG DELIVERY SYSTEM:
Painless technology not only plays important role in delivering insulin but also it is used in delivering drugs in flu, hepatitis-B vaccine, growth hormone, female hormones and some antibiotics which are not suitable for oral delivery. A review says “one million people every year suffer from injury or infection from hypodermic needles, requiring treatment from a health care professional.”
Needle free injectors have been developed to resolve the issue of pain and fear and hence to improve compliance. There are two types of needle free injectors-fluid and powder. Needle free powder injections have been utilized for the delivery of vaccine for hepatitis B, aprostadil for erectile dysfunction, the anaesthetic lidocaine, a granulocyte macrophages colony stimulating factors tumor vaccine for malignant melanoma and DNA coated particles. Also needle free injection devices for subcutaneous delivery of rhGH could minimize discomfort.
CONCLUSION:
Needle-free technology offers the very obvious benefit of reducing patient concern about the use of needle. Additional benefits include very fast injection compared with conventional needle and no needle disposal issues. Not only it can benefit the pharmaceutical industry in increasing product sales, it has the added potential to increase compliance with dosage regimens and improved outcomes. In the developing world, there are major challenges of disease transmission through re-use of needles. Organisations such as WHO and CDC (Centre for Disease Control) and groups like Gates Foundation have supported the development of needle-free alternatives for drug delivery.
REFERENCES:
1. Verge D. Insulin: New molecules and routes of administration-Biotechnological innovations
in insulin therapy, Medical Science 2004, 20, 986-998.
2. Koda-Kimble MA, Young LY, Kradjan WA, Joseph B, Allodredge BK ; Diabetes Mellitus ; In: Applied Therapeutics- The clinical use of drug, Philapedia, Corelli Lippincott and Willkins:2004 ;13,50.
3 . Kuzuya, T, Nakagawa S, Satoh J, KanazawaY, IwamotoY, KobayashiM, Nanjo K,Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, and Kadowaki T, Diabetes Res. Clinical Practice,2002.p.55, 65.
4. Heinemann L ; Overcoming obstacles:new management options; Eur J.Endocrinol , 2004 Oct; 151 Suppl 2:T 23-7; discussion T 29-30.
5Images.google.co.in/images?hl+en&g=+devices+used+in+needle+free+drug+ delivery + system & btnG=search+images&ghv=2.
6. https://en.wikipedia.org/wiki/insulin.
7.Satyanarayana U,Chakrapani U; Recombinant DNA & Biotechnology ; In: Biochemistry, ,3rd ed.Kolkata , Books & Allied(P)Ltd , 2006 ; 607,676.
8.Lehinger, David LN, Cox MC; Hormones : Diverse structure for diverse functions; In: Principle of biochemistry, 4th ed. New York, W.H.Freemon and company, 2007, 887.
9. pharmainfo.net/reviews/alternatives_insulin_delivery_system.
10. en.wikipedia.org/wiki/jet_injector.
11. Goodman & Gillman, Hardman JG, Limbird LE, Goodman and Gillman A; Insulin, oral hypoglycemic agent, and pharmacology of the endocrine pancreas; In: The Pharmacological basis for therapeutics, 12th ed.USA, McGrow-Hill medical publishing division, 2005, 1679-1701
12.wikipedia.org/wiki/insulin-therapy_inhaler.
13.wikipedia.org/wiki/insulin-spray
14.wikipedia.org/wiki/insulin-pen
15.novolog.com/devices_flexpen.asp?s=ds&h=60
16.islet.isletsofthope.com/pic/zzpic insulin pen .jpg
17.flamel.technology.com
18.wikipedia.org/wiki/insulin-port.
19.port=images.google.co.in/images?gbv=2&hl=en&q=insulin+port&btnG=Search+iages.
20.wikipedia.org/wiki/transdermal-patch.
21.wikipedia.org/wiki/special:search?Search=insulin+transdermal+patch.
22.pharmainfo.net/reviews/recent_progress_active_transdermal_drug_delivery.
23.pump=Images.google.co.in/images?gbv=2&hl=en&q=insulin_pump_with_infusion_set.jpg&imgrefurl=search+images.
24.debiotech.com
25.en.wikipedia.org/wiki/insulin-pills.
26.en.wikipedia.org/wiki/insulin-capsules.
27. in.search.yahoo.com/search?fr=yfp_t_in&type=ds&p=insulin+capsule.
28. healthworld.blogspot.com/2008//11/artificial.pancreas.btm&usg.
29.pharmainfo.net/reviews/pancreas_transplantation_insulin_free_novel_free_ therapy _treating_type_1_diabetes.
30. diabetes.niddk.nil.gov/DM/pubs/insulin.
31.en.wikipedia.org/wiki/insulin-analouge.
32. Bhattacharya SK, Sen P, Ray A, Das PK; Endocrine system, In: Pharmacology, 2nd ed, New Dehli, Elsevier Publication, 2004, 356.
33.https://en.wikipedia.org/wiki/insulin-complement.
34.Ahuja S, Stephen S; New drug delivery systems; In: Handbook of Modern pharmaceutical analysis, vol-3, New York ,Separation Science and technology, 2001, 319-320
35.medindia.net/news/view news main. asp=x=17422
36.pharmainfo.net/in_pharmatechnologist.com/packaging/micro.jet_for _painless_needlefree_ijection.
37.novonordiskcare.com/insulin therapy.
38.jbc.org/cgi/content/citation/70/1/89
39.elan.com
40.port.technology
41.durect.com
42.lifeclinic.com
Reference ID: PHARMATUTOR-ART-1023
FIND OUT MORE ARTICLES AT OUR DATABASE
|
Type |
Method |
Advantages |
Disadvantages |
|
Needle and syringe |
Injection |
Inexpensive; insulin can be mixed |
Less convenient than some other methods; needles make some patients uncomfortable |
|
Insulin pump |
Automatic release via battery-run pump |
Insulin delivered easily; fewer needle sticks |
Expensive; frequent glucose testing is necessary |
|
Insulin pen |
Injection |
Convenient and discreet storage and delivery |
Not available for all insulin types |
|
Insulin jet injector |
High-pressure air |
No needles involved |
Cost; some patients find high-pressure air painful; some medication does not penetrate skin |
|
Table no.(1): Comparison between painful and painless insulin drug delivery system |
|||
|
Technology Name |
Company Name |
Status |
Description |
|
|
Implaject |
Caretek Medical |
Under Clinical Trials |
Simple, hand-held needle free injection device. Can be configured to be reusable with disposable cartridges. |
|
|
Crossject |
Crossject |
Under development |
Prefilled, single use disposable NFI. Uses chemical reaction togenerate propellant at the time of administration |
|
|
PowderJect |
PowderMed |
Marketed |
It painlessly delivers DNA vaccines to the skin in a dry formulation |
|
|
Zoma-jet 2 Vision |
Antares Pharma |
Launched in Europe in 2003 |
Customized version ofMedi-jector vision licensed to Ferring for administration of their human growth hormone, Zomacton for distribution in Europe |
|
|
Valeo (MJ8) |
Antares Pharma |
Clinical Trials completed, availablefor licensing |
Next generation pen-style, spring-powered device. Designed for use with drugs in cartridge containers, rather than vials. |
|
|
Injex 30 |
Injex (HNS International) |
Marketed in US |
Spring-powered hand-held device with disposable ampoules that delivers 0.05-0.3 ml. Focused on insulin delivery |
|
|
mhi-500 |
The Medical House/Bioject |
Marketed in Europeas an insulin delivery device for insulin delivery. It system |
Spring-powered hand-held delivers volume in the range of 0.02-0.5 ml |
|
|
Table no.(2): Marketed preparations |
||||









