About Authors:
Pratapwar A.S1*, Agrawal V.A2
S.N.Institute of Pharmacy Pusad, Yewatmal
Corresponding Author: Agrawal V.A
vijayagrawal499@gmail.com
Abstract:
Out of all the routs of drug administration oral is the most convenient and better for self administration from the patient point of view and suitable for the release controlled delivery systems from the formulators view. In the today’s field of modern drug therapy much more attention is given on the development of oral release modified delivery systems such as programmed release, gastro retentive, floating and most important pulsatile or time programmed release systems. The chronotherapeutic drug delivery systems are designed to maintain the adequate drug concentration according to the needs of the physiological states of patient’s body and the cardian rhythm. Pulsatile release systems are designed to deliver the drug at the right site, at right time and in right concentration after a predetermined lag time. To achieve the desired pulse release pattern, many technological approaches have been investigated like Single unit system,Tablet-Time clocks system, Capsule- Pulsincap system and multiple unit system, Pellets,Time controlled explosion system,Multi-layered Tablet,Chemical stimuli induced pulsatile systems like Glucose-responsive insulin release devices, Inflammation-induced release, intelligent gels responding to antibody concentration, release control by use of the soluble, rupturable, swelling and erodible polymers in the formulation. The present article focus on the basics of chronotherapeutic drug delivery systems, diseases with cardian rethyms, need, advantages, types and approaches for the development of Pulsatile drug delivery systems.
REFERENCE ID: PHARMATUTOR-ART-1640
INTRODUCTION
The development of novel technologies in the pharmaceutical field, drug delivery systems have drawn an increasing interest over the last few decades. Nowadays, the emphasis of pharmaceutical research is turned towards the development of more efficacious drug delivery systems with already existing molecule rather going for new drug discovery because of the inherent hurdles posed in drug discovery and development process 1. This challenge has been met by a wide range of techniques, including osmotically driven pumps2, matrices with controllable swelling 3, diffusion4 or erosion rates 5and multi-layered matrices6.
Oral controlled release and time programmed drug delivery systems represent the most popular form of controlled drug delivery systems for the obvious advantages of oral route of drug administration. Such systems release the drug with constant or variable release rates. These dosage forms offer many advantages, such as nearly constant drug level at the site of action, prevention of peak valley fluctuations, reduction in dose of drug, reduced dosage frequency, avoidance of side effects, improved patient compliance and thus the better therapeutic outcome of the medication.
Pulsed or pulsatile drug release is defined as the rapid and transient release of a certain amount of drug molecules with in a short time-period immediately after a predetermined off-release period or A pulsatile drug delivery system is characterized by a lag time that is an interval of no drug release followed by rapid drug release7.
Pulsatile drug delivery system is desirable for the drugs acting or having an absorption window in the gastro-intestinal tract or for the drugs with an extensive first pass metabolism Ex, B-blockers or for the drugs, which develop biological tolerance, where the constant presence of drug at the site of action diminishes the therapeutic effect or for drugs with special pharmacokinetic features designed according to the circadian rhythm of human. Sustain release dosage forms may maintain nearly constant plasma drug concentration in therapeutic window for prolonged time. Pulsatile release dosage forms release drug in pulsatile manner and maintain plasma drug level within therapeutic range 7.
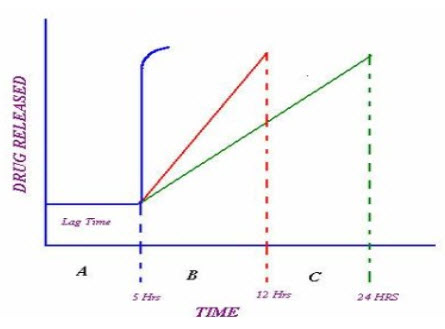
FigureNo.1: Drug release profile of pulsatile drug delivery system 8, 9
A: Ideal sigmoid release. B & C: Delayed release after initial lag time
The first pulsed delivery formulation that released the active substance at a precisely defined time point was developed in the early1990s. In this context, the aim of the research was to achieve a so called sigmoidal release pattern (pattern A in Figure 1). The characteristic feature of the formulation was a defined lag time followed by a drug pulse with the enclosed active quantity being released at once. Thus, the major challenge in the development of pulsatile drug delivery system is to achieve a rapid drug release after the lag time. Often, the drug is released over an extended period of time also achieved. (Patterns B&C infigure1)
Circadian rhythm
The dependence of several diseases and body function on circadian rhythm is well known. A genetic control of a “master clock” located in the nucleus supra-chiasmaticus has been recently proposed. Numerous studies conducted, suggest that pharmacokinetics, drug efficacy and side effects can be modified by followingtherapy matching the biological rhythm. Specificity in delivering higher amount of drug in a burst at circadian timings correlated with specific pathological disorder is a key factor to achieve maximumdrug effect 10 - 12. Particular rhythms in the onset and extent of symptoms were observed in diseases such as, bronchial asthma, myocardial infarction, angina pectoris, rheumatic disease, ulcer, diabetes, attention deficit syndrome, hyper-cholesterolemia and hypertension 13
Necessity of Pulsatile Drug Delivery Systems
There are many conditions and diseases where sustained release formulations do not show good efficacy. In such cases Pulsatile DDS is applicable.
* Many body functions follow circadian rhythm, i.e., their activity increases or decreases with time. A number of hormones like rennin, aldosterone, and cortisol show daily as well as timely fluctuations in their blood levels. Circadian effects are also observed in case of pH and acid secretion in stomach, gastric emptying, and gastro-intestinal blood transfusion 14.
* Severity of diseases like bronchial asthma, myocardial infarction, angina pectoris, rheumatic disease, ulcer andhypertension is time dependent 15. Sharp increase in asthmatic attacks during early morning hours have been reported 16. Such a condition demands supplement of drug at particular time rather than maintaining constant plasma drug level. A drug delivery system administered at bedtime, but releasing drug as a burst after the time of administration (during morning hours), would be ideal in this case. Same is true for preventing heart attacks in the middle of the night and the morning stiffness typical of people suffering from arthritis.
* Some drugs (e.g. Salbutamol sulphate) produce biological tolerance and hence demand for a system that will prevent their continuo us presence at the site of action as this tends to reduce their therapeutic effect 17.
* Protection from gastric environment is essential for the drugs that undergo degradation in gastric acidic medium (e.g., peptide drugs), irritate the gastric mucosa(NSAIDS) or induce nausea and vomiting. These conditions can be satisfactorily handled by enteric coating, and in this sense, enteric coating can be considered as a pulsatile drug delivery system.
* To achieve localized action at distal organs of GIT such as colon for drugs used in ulcerative colitis (e.g. Sulfasalazine) the drug release needs to be prevented in the upper two-third portion of the GIT 18.
* The drugs that undergo extensive first-pass metabolism (ß-blockers) and those that are characterized by idiosyncratic pharmacokinetics or pharmacodynamics resulting in reduced bioavailability, altered drug/metabolite ratios, altered steady state levels of drug and metabolite, and potential food-drug interactions require delayed release of the drug to the extent possible 9.
All of these conditions demand for an efficiently programmed drug delivery system releasing the right amount of drug at the right time. This can be achieved by Pulsatile Drug Delivery Systems.
Advantages of pulsatile drug delivery system 9, 10
* Many body functions that follow circadian rhythm. A number of hormones like rennin, aldosterone, and cortisol show daily fluctuations in their blood levels. Circadian effects are also observed in case of pH and acid secretion in stomach, gastric emptying, and gastro-intestinal blood transfusion.
* Diseases like bronchial asthma, myocardial infarction, angina pectoris, rheumatic disease, ulcer, and hypertension display time dependence. Sharp increase in asthmatic attacks during early morning hours. Such a condition demands considerations of diurnal progress of the disease rather than maintaining constant plasma drug level. A drug delivery system administered at bedtime, but releasing drug well after the time of administration (during morning hours), would be ideal in morning stiffness typical of people suffering from arthritis.
* Drugs that produce biological tolerance demand for a system that will prevent their continuous presence at the biophase, as this tends to reduce their therapeutic effect.
* The lag time is essential for the drugs that undergo degradation in gastric acidic medium (e.g., peptide drugs) irritate the gastric mucosa or induce nausea and vomiting. These conditions can be satisfactorily handled by enteric coating, and in this sense, enteric coating can be considered as a pulsatile drug delivery system.
* Targeting a drug to distal organs of gastro-intestinal tract (GIT) like the colon requires that the drug release be prevented in the upper two-third portion of the GIT.
* The drugs that undergo extensive first-pass metabolism (β-blockers) and those that are characterized by idiosyncratic pharmacokinetics or pharmacodynamics resulting in reduced bioavailability, altered drug/metabolite ratios, altered steady state levels of drug and metabolite, and potential food-drug interactions require delayed release of the drug to the extent possible.
Classification of pulsatile drug delivery systems
Pulsatile drug delivery systems (PDDS) can be classified in site-specific and time-controlled systems.
A. Pulsatile drug delivery system
a. Time specific
1. Single unit system:Tablet-Time locks system
:Capsule-Pulsincap system
2. Multiple unit system: Pellets,Time controlled explosion system.
b. Site specific
Time controlled delivery system
The principle of time controlled drug delivery systems is that the release of the drug happens according to a predetermined rate so to achieve maximum therapeutic and minimum toxic effect. Systems having a lag phase (delayed release systems) and systems where the release is following a biological circadian rhythm are the most commonly used controlled release systems. Most delayed release delivery systems are reservoir devices covered with a barrier coating, which dissolves, erodes or ruptures after a lag phase.Well-known coating technique employs a water-permeable but insoluble film which encloses the active ingredient and an osmotic agent. As water from the gut slowly diffuses through the film into the core, the core swells until the film bursts, releasing the drug19.
Single unit system
Capsule Based (e.g., Pulsincap® system)
Amidon and Leesman described a drug delivery system for administering a drug in controlled pulse doses to an aqueous environment in the body of a living being.
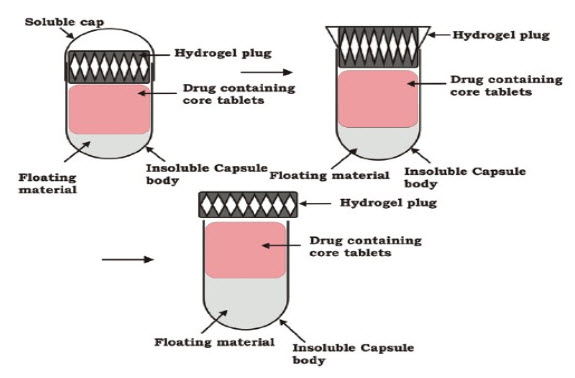
FigureNo. 2: Schematic diagram of capsular system
Percel and coworkers43 described a capsule capable of delivering therapeutic agents in the body in a time-controlled or position-controlled pulsatile release fashion, composed of one or more populations of multicoated particulates (beads, pellets, granules, etc.). Each bead has been prepared by coating an inert particle such as a non-pareil seed (sugar sphere), with a drug and a polymeric binder or by preparing a drug containing particle by granulation and/or extrusion-spheronization, coating the active drug particle with a plasticized enteric coating, and coating plasticized enteric coated drug particle with a mixture of a water insoluble polymer and an enteric polymer. One of themembrane barriers is composed of an enteric polymer while the second membrane barrier is composed of a mixture of water insoluble polymer and an enteric polymer. The composition and the thickness of the polymeric membrane barriers determine the lag time and duration of drug release from each of the bead populations. Optionally, an organic acid containing intermediate membrane may be applied for further modifying the lag time and/or the duration of drug release 20.
* The Pulsincap® system
A general structure of such systems consists of an insoluble capsule body containing a drug and a plug. The plug may be erodible, swelling or soluble which is removed after a predetermined lag time.The Pulsincap® system is an example of such a system that is made up of a water-insoluble capsule body filled with drug formulation 21-23. The body is closed at the open end with a swellable hydrogel plug. Upon contact with dissolution medium or gastro-intestinal fluids, the plug swells and after a lag time, pushes itself out of the capsule. This leads to drug release as a pulse. The lagtime can be controlled by manipulating the dimension and the position of the plug. For water-insoluble drugs, a rapid release can be ensured by incorporation of disintegrants or effervescent agents. The plug material consists of insoluble but permeable and swellable polymers (e.g., polymethacrylates), erodible compressed polymers (e.g., hydroxypropylmethyl cellulose, polyvinyl alcohol, polyethylene oxide) congealed melted polymers (e.g., saturated polyglycolated glycerides, glyceryl monooleate), and enzymatically controlled erodible polymer (e.g., pectin,agar) 24.
Osmotic based pump capsule(e.g., Port® System)
Osmotic delivery capsules ("osmotic pumps") function by virtue of walls which selectively pass water into the capsule reservoir. Absorption of water by the capsule through these walls is driven by a water-attracting agent in the capsule interior which creates osmotic pressure across the capsule wall andthe structure of the capsule wall does not permit the capsule to expand, and as a result, the water uptake causes discharge of the beneficial agent through an orifice in the capsule at the same rate that water enters by osmosis25.
* The Port® System
The Port®System consists of a gelatin capsule coated with a semipermeable membrane (e.g., cellulose acetate). Inside the capsule were an insoluble plug and an osmotically active agent along with the drug formulation26. When this capsule came in contact with the dissolution medium, water diffuses across the semipermeable membrane, resulting in increased pressure inside that ejects the plug after a predetermined lag time. The lag time is controlled by coating thickness.
Tablet based
Most of the pulsatile drug delivery systems are reservoir devices coated with a barrier layer. This barrier erodes or dissolves after a specific lag time, after that the drug is released rapidly. The lag time depends on the thickness of the coating layer.
The Time Clock® system
This system consists of a solid dosage form coated with lipidic barriers containing carnauba wax and bees’ wax along with surfactants, such as span 8027-28. After a lag time proportional to the thickness of the film, this coat erodes or emulsifies in the aqueous environment, and the core is then available for dispersion. A study with human volunteers has shown that the lag time was independent of gastric residence time, and the hydrophobic film re-dispersion did not appear to be influenced by mechanical action of stomach or gastro-intestinal pH or the presence of intestinal enzymes. The lag time increased withincreasing coating thickness. Such systems are better suited for water-soluble drugs. The major advantage of this system is its ease of manufacturing without any need of special equipment. However, such lipid-based systems may have high in-vivo variability.
The Chronotropic® system
This system consists of a drug-containing core coated by hydroxypropylmethyl cellulose (HPMC), a hydrophilic swellable polymer, which is responsible for a lag phase in the onset of release. In addition, by coating the system by enteric polymer such ascellulose acetate phthalate, the variability in gastric emptying time can be overcome, and a colon-specific release can be obtained, assuming small intestinal transit time is not changed 29. The lag time is controlled by the thickness and the viscosity grades of HPMC. The system is suitable for both tablets and capsules.
Multi-layered Tablet
A release pattern with two pulses was obtained from a three-layered tablet containing two drug containing layers separated by a drug-free gellable polymeric barrier layer 30-31. This three-layered tablet was coated on three sides with impermeable ethyl cellulose, and the top portion was left uncoated. On contact with dissolution medium, the initial dose incorporated into the top layer was released rapidly from the non-coated surface. The second pulse was obtained from the bottom layer after the gelling barrier layer of HPMC was eroded and dissolved. The appearance of the second pulse was controlled by the rate of gelling and/or dissolution of the barrier layer. The gelling polymers reported include cellulose derivatives like HPMC, methyl cellulose, some classes of methacrylate’s (Eudragits®) or polyvinyl alcohols of various molecular weights and the coating materials include ethyl cellulose, cellulose-acetate-propionate, methacrylic polymers, acrylic and methacrylic co-polymers, and polyalcohol’s32. A marketed product of this class is SyncroDose™.
Multiparticulate Systems
Multiparticulate systems (e.g., pellets, beads) offer various advantages over single-unit systems 33. These systems have no risk of dose dumping, they provide flexibility of blending units with different release patterns, and provide reproducible and short gastric residence time. But the drug-carrying capacity of multiparticulate systems is lower due to presence of higher quantity of excipients. Such systems are a reservoir type with either rupturable or altered permeability coating and generally housed in capsular body.
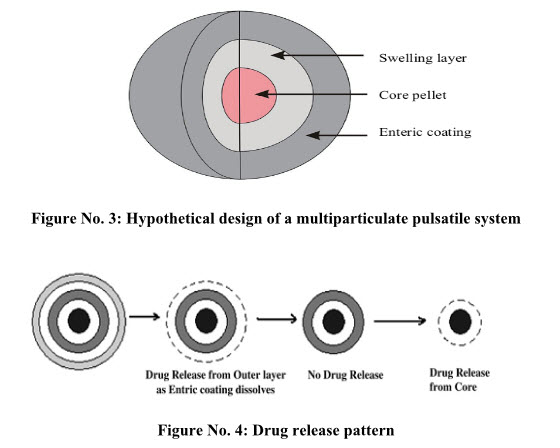
System Based on Rupturable Coating
Time-Controlled Explosion System
This type of system is multiparticulate system in which drug is loaded through coating on non-pareil sugar seeds followed by a swellable layer and an insoluble top layer 34,35. Superdisintegrants like sodium carboxymethylcellulose, sodium starch glycolate, L-hydroxypropyl cellulose are used as swelling agents. Coating polymers used are like polyvinyl acetate, polyacrylic acid, polyethylene glycol, etc. are used. Alternatively, an effervescent system comprising a mixture of tartaric acid and sodium bicarbonate may also be used. Upon coming in contact with water, the swellable layer expands, resulting in rupture of film coat with subsequent rapid drug release.
Osmotic-Based Rupturable Coating Systems
Permeability Controlled System
This system is based on a combination of osmotic and swelling effects. This system contains a core containing the drug, a low bulk density solid and/or liquid lipid material (e.g., mineral oil) and a disintegrants. This core is then coated with cellulose acetate. Upon immersion in aqueous medium, water penetrates the core displacing lipid material. After the depletion of lipid material, internal pressure increases until a critical stress is reached, which results in rupture of coating 36.
Delivery by Change in Membrane Permeability
The permeability and water uptake of acrylic polymers with quaternary ammonium groups (e.g. Eudragit RS 30D) can be influenced by the presence of different counter-ions in the medium 37. The ammonium group being hydrophilic facilitates the interaction of polymer with water, and hence changes its permeability and allows water to permeate the active core in a controlled manner. This property is essential to achieve a precisely defined lag time.
Site Specific System or Stimuli induced pulsatile release system
Several polymeric delivery systems undergo phase transitions and demonstrate marked swelling-deswelling changes in response to environmental changes including solvent composition, ionic strength, temperature, electric fields, and light 38. Responsive drug release from those systems results from the stimuli-induced changes in the gels or in the micelles, which may deswell, swell, or erode in response to the respective stimuli.
Chemical stimuli induced pulsatile systems
Glucose-responsive insulin release devices
In case of Diabetes mellitus there is rhythmic increase in the levels of glucose in the body, requiring injection of the insulin at proper time. Several systems have been developed which are able to respond to changes in glucose concentration. The system includes pH sensitive hydrogel containing glucose oxidase immobilized in the hydrogel. When glucose concentration in the blood increases glucose oxidase converts glucose into gluconic acid which changes the pH of the system. This pH change induces swelling of the polymer which results in insulin release. Examples of the pH sensitive polymers include N, N-dimethylaminoethyl methacrylate, chitosanand polyoletc 39, 40.
Inflammation-induced pulsatile release
On receiving any physical or chemical stress, such as injury, fracture etc., inflammation take place at the injured sites. During inflammation, hydroxyl radicals are produced from these inflammation-responsive cells. Degradation via hydroxyl radicals however, is usually dominant and rapid when Hyaluronic acid gel is injected at inflammatory sites. Thus, it is possible to treat patients with inflammatory diseases like rheumatoid arthritis; using anti-inflammatory drug incorporated HA gels as new implantable drug delivery systems 41.
Drug release from intelligent gels responding to antibody concentration
There are numerous kinds of bioactive compounds which exist in the body. Recently, novel gels were developed which responded to the change in concentration of bioactive compounds to alter their swelling/deswelling characteristics. Special attention was given to antigen-antibody complex formation as the cross-linking units in the gel, since such interaction is very specific. Utilizing the difference in association constants between polymerized antibodies and naturally derived antibodies towards specific antigens, reversible gel swelling/deswelling and drug permeation changes occurs
pH sensitive drug delivery system
This type of PDDS contains two components. The first is fast release type while the other is pulsed release which releases the drug in response to change in pH. Examples of pH dependent polymers include cellulose acetate phthalate, polyacrylates, and sodium carboxymethylcellulose. These polymers are used as enteric coating materials so as to provide release of drug in the small intestine 42.
External stimuli induced pulsatile release
Electro responsive pulsatile release
Electrically responsive delivery systems are prepared from polyelectrolytes (polymers which contain relatively high concentration of ionisable groups along the backbone chain) and are thus, pH-responsive as well as electro-responsive. Examples of naturally occurring polymers include hyaluronic acid, chondroitin sulphate, agarose, carbomer, xanthan gum and calcium alginate. The synthetic polymers are generally acrylate and meth acrylate derivatives such as partially hydrolyzed polyacrylamide, polydimethylaminopropyl acrylamide 43.
Micro electro mechanical systems (MEMS)
A micro fabricated device has the ability to store and release multiple chemical substances on demand by a mechanism devoid of moving its parts 44, 45. The digital capabilities of MEMS may allow greater temporal control over drug release compared to traditional polymer-based systems. The prototype microchip is made of silicon and contains a number of drug reservoirs, each reservoir is sealed at one end by a thin gold membrane of material that serves as an anode in an electrochemical reaction and dissolves when an electric potential is applied to it in an electrolyte solution. The reservoirs are filled with any combination of drug or drug mixtures in any form (i.e. solid, liquid or gel). When release is desired, an electric potential is applied between an anode membrane and a cathode, the gold membrane anode dissolves within 10- 20 seconds and allows the drug in the reservoir to be released.
Magnetically induced pulsatile release
The use of an oscillating magnetic field to modulate the rates of drug release from polymer matrix was one of the old methodologies. Magnetic carriers receive their magnetic response to a magnetic field from incorporated materials such as Magnetite, Iron, Nickel, Cobalt etc. For biomedical applications, magnetic carriers must be water-based, biocompatible, non-toxic and non-immunogenic mechanistic approach based on magnetic attraction is the slowing down of oral drugs in the gastrointestinal system. This is possible by filling an additional magnetic component into capsules or tablets. The speed of travel through the stomach and intestines can then be slowed down at specific positions by an external magnet, thus changing the timing and/ or extent of drug absorption into stomach or intestines 46.
Conclusion:
Pulsatile delivery systems are gaining much more importance in the field of time programmed release drug delivery systems due to the potential advantages, improved patient compliance and better therapeutic outcome. The hurdles in the development of robust manufacturing and processing technologies need to be studied and circumvented. Disease specific drug delivery systems need to be developed.In nutshell the time programmed release delivery systems can prove more beneficial than the conventional systems in the era of modern therapies for the patient. Rather than searching the new drugs for the treatment, release modified systems can save much more time of drug discovery process. Better therapeutic outcome of the existing drug molecules can be taken by formulating in the form of time programmed release or pulsatile drug delivery systems.
REFERENCES
1. 1. SurvaseS. Kumar N. Pulsatile Drug Delivery: Current Scenario. Current Research & Infor. Pharm. Sci., 2007, 8 (2): 27-33.
2. LiuL. X., WangX. C. Solubility-modulated monolithic osmotic pump tablet for atenolol delivery. Eur. J. Pharm. Biopharm., 2008, 68: 298-302.
3. ConteU. Maggi L. A flexible technology for the linear, pulsatile and delayed release of drugs allowing for easy accommodation of difficult in vitro targets. J. Control. Release, 2000, 64: 263-68.
4. Lee E. S., KimS. W., S. H. Kim. et al. Drug release from hydrogel devices with rate-controlling barriers. J. Membr. Sci. 1980, 7: 293-303.
5. McConvilleJ. T., RossA. C., FlorenceA. J., et al. Erosion characteristics of an erodible tablet incorporated in a time-delayed capsule device, Drug Dev. Ind. Pharm., 2005, 31: 79–89.
6. LuS., AnsethK., Polymerization of multilaminated poly (HEMA) hydrogels for controlled release. J. Control. Release, 1999, 57: 291-300.
7. Kikuchi A. Okano T. Pulsatile drug release control using hydrogels. Advan. Drug Del. Reviews, 2002, 54: 53-77.
8. Gothoskar A.V., Joshi A.M., Joshi N.H. Pulsatile drug delivery systems: a review. Drug Delivery Technology 2004; 4(5):1-11.
9. Shivakumar H.G., Pramod kumar T.M., Kashppa G.D. Pulsatile drug delivery system, Indian J Pham Educ 2003;37(3):125
10. LemmerB., The clinical relevance of chronopharmacology in therapeutics. Pharmacol. Res.1996, 33: 107-115.
11. Björm Hemmer. Circadian rhythms and drug delivery. J. Control. Release, 1991, 16:63-74.
12. LemmerB., Chronopharmacokinetics: implications for drug treatment, J. Pharm. Pharmacol., 1999, 51 (8): 887-890.
13. Roy P., ShahiwalaA. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. J. Control. Release, 2009, 134:74-80.
14. Drayer J.I., Weber M.A., Nakamura D.K., Automated ambulatory blood pressure monitoring: a study in age-matched normotensive and hypertensive men, Am Heart J 1985;109, 1334–1338.
15. Goo R.H., Moore J.G., Greenberg E., Alazraki N.P. Circadian variation in gastric emptying of meals in humans. Gastroenterol. 1987; 93(3):515-518.
16. Lemmer B. Chronopharmacokinetics : implications for drug treatment. J Pharm. Ph armacol. 1999; 51:887-890.
17. Dethlefsen U., Repges R. Ein neues therapieprinzip bei nächtlichem asthma. Med Klinik. 1985;80:44-47.
18. Chang R.K, Guo X., Burside B.A., Couch R.A., Rudnic EM. Formulation approaches for oral pulsatile drug delivery. Amer Pharma Rev. 1999;2(1):51-57.
19. Gazzaniga A., Sangalli M.E., Giordano F. Oral chronotopic & Mac226: drug delivery systems: achievement of time and/or site specifity. Eur J Biopharm. 1994; 40(4):246-250.
20. Sharma V.K., Hussain J., Khorakiala H.F., Composition for pulsatile delivery of diltiazem and process of manufacture, 6635277, (2003).
21. Percel P.J., Vishnupad K.S.,Venkatesh G.M., Lee D.Y., Timed Pulsatile Drug Delivery Systems, US20067048945 (2006)
22. Saeger H., Virley P. Pulsincap& Mac226: Pulsed-Release Dosage Form. Product information from Scherer DDS, Ltd; 2004.
23. Wilding I.R., Davis S.S., BakhshaeeM., Stevens HNE, Sparrow R.A., Brennan J. Gastrointestinal transit and systemic absorption of captopril from a pulsed-release formulation. Pharm Res. 1992; 9:654-657.
24. McNeil M.E., Rashid A., Stevens HNE. Dispensing Device. WO Patent No. 90/09168 (1990).
25. Krögel I., Bodmeier R. Pulsatile drug release from an insoluble capsule body controlled by an erodible plug. Pharm Res. 1998;15(3):474-481.
26. Krögel I., Bodmeier R. Evaluation of an enzyme-containing capsular shaped pulsatile drug delivery system. Pharm Res. 1999;16(9):1424-1429.
27. Jenkins S., Liversidge G., modified release compositions comprising a fluorocytidine derivative for the treatment of cancer, wo2006110800 (2006)
28. Crison J.R., Siersma P.R., Taylor M.D., Amidon G.L. Programmable oral release technology, Port Systems & Mac226: a novel dosage form for time and site specific oral drug delivery. Proceed Intern Symp Control Rel Bioact Mater. 1995;22:278-279.
29. Pozzi F., Furlani P. Orale Feste Pharmazeutische Darreichungsform Mit Programmierter Freisetzung. DE Patent No. 4122039; 1992.
30. Wilding I.R., Davis S.S., Pozzi F., Furlani P., Gazzaniga A. Enteric coated timed release systems for colonic targeting. Int J Pharm.1994;111:99-102.
31. Poli S., Busetti C., Moro L. Oral Pharmaceutical Composition for Specific Colon Delivery. EP Patent No. 0,572,942; 1993.
32. Conte U., Giunchedi P., Maggi L., Sangalli M.E., Gazzaniga A., Colombo P., LaManna A. Ibuprofen delayed release dosage forms: a proposal for the preparation of an in vitro/in vivo pulsatile system. Eur J Pharm.1992;38(6):209-212.
33. Conte U., La Manna A., Colombo P. Tablet for Pharmaceutical Use Able to Release Active Substances At Successive Times. US Patent No. 4,865,849; 1989.
34. Daumesnil R. Marketing Considerations for multiparticulate drug delivery systems. In:Ghebre-Sellassie I, ed. Multiparticulate Oral Drug Delivery. New York, NY: Marcel Dekker, Inc.; 1994:457-474.
35. Ueda S., Yamaguchi H, Kotani M., Kimura S., Tokunaga Y., Kagayama A., Hata T. Development of a novel drug release system, time-controlled explosion system (TES). Part II: design of multiparticulate TES and in vitro drug release properties. Chem Pharm Bull. 1994; 42(2):359-363.
36. Ueda Y., Hata T., Yamaguchi H., Kotani M, Ueda S. Developmentof a novel drug release system, time-controlled explosion system (TES). Part 1: concept and design. J Drug Targeting. 1994;2:35-44.
37. Amidon G.L., Leesman G.D. Pulsatile Drug Delivery System.US Patent No. 5,229,131; 1993.
38. Bodmeier R., Guo X., Sarabia R.E., Skultety P. The influence of buffer species and strength on diltiazem HCl release from beads coated with aqueous cationic polymer dispersions, Eudragit RS, RL 30D. Pharm Res.1996;13(1):52-56.
39. Sershen S.R., Westcott S.L., Halas N.J., West N.J. Temperature sensitive Polymer-nano shell composites for photo thermally modulated drug delivery. J Biomed Mater Res 2000; 293-298.
40. Gutowska A., Bark J.S., Kwon I.C., Bae Y.H., Kim S.W. Squeezing hydrogels for controlled oral drug delivery. J Control Rel 1997; 48: 141-148.
41. Yui N., Okano T., Sakurai Y. Inflammation Responsive Degradation of Cross-Linked
42. Hyaluronic-Acid Gels. J Control Rel 1992; 22:105-116
43. Miyata T, Asami N, Uragami T. A reversibly antigen-responsive hydrogel. Nature1999; 399:766-769.
44. Sanvase S., Neeraj K. Pulsatile drug delivery: Current scenario.CRIPS 2007;8:27-33.
45. Santini J.T., Cima M.J., Langer R. A controlled-release microchip. Nature 1999; 335-338.
46. Santini J.T., Richards A.C., Schiedt R., Cima M.J., Langer R. Microchips as controlled-drug delivery devices. Angew Chem Int Ed 2000; 2396-2407.
47. Chen H., Langer R. Magnetically-responsive polymerized liposomes as potential oral delivery vehicles. Pharm Res 1997; 537-540.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









