 About Authors:
About Authors:
*Kalpesh Ashara, Jignesh Solanki
B.K.Mody Govt.Pharmacy College Rajkot,
Department of Pharmaceutics,GTU, Gujarat, India.
*kalpeshshr5@gmail.com
Abstract:
Microspheres are solid spherical particles containing a dispersed drug in organic solution fall in a range of 1-1000mm. Microspheres or micro particles are monolithinic device refer to a rate controlling matrix throughout the drug is dissolved or dispersed, while microcapsules are device which consists of cell-like dosage forms with the drug contain within the rate controlling membrane. Microspheres are prepared by several methods. Here Microspheres are prepared using a surface active agent SLS. Then Evaluation of Microspheres is carried out by means of several parameters. Then concluded that the diclofenac: polymer ratio of 1:2 & organic solvent (MeOH: DCM) ratio of 1:4 was found to be optimum for spherical shape of microspheres as well as Practical Yield.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1499
Introduction:
Microspheres are solid, spherical particles containing a dispersed drug in organic solution fall in range of 1-1000mm.The first pharmaceutical consisting of microsphere was controlled release aspirin product. Microspheres or micro particles are the examples of monolithic devices refer to a rate-controlling matrix throughout the drug is dissolved or dispersed. While microcapsules are a device, which consists of shell like dosage form with, the drug contained within a rate controlling membrane.
Microspheres are used for sustained and controlled release.e.g. Drugs like riboflavin, indomethacin, aspirin and steroids like progesterone, testosterone, etc. can be incorporated in it to control their release.It are used in enteric release dosage form.e.g. Drugs like aspirin, salbutamol sulphate which irritant to the stomach and other side effects can be incorporated in microspheres for their selective release in intestine. It is used for drug targeting.e.g. Casein and gelatin microspheres containing Adriamycin were magnetically delivered to the tumor site. It is used as antigens carrier.e.g. Microspheres prepared from poly (lactic acid) and its copolymer with glycolic acid (PLGA) of varying composition has been used to improve the ability of the antigens to provoke a mucosal immune response. To alter the residence time and to improve the bioavaibility.e.g. Albumin and gelatin microspheres containing pilocarpine nitrate (ophthalmic drug delivery) for delivery to eye increase residence time of drug in the eye and provide improved bioavaibility. It is used to protect reactive materials against environment.e.g. Vitamins, aspirin. To separate incompatible substances.e.g. The stability of incompatible drugs like aspirin and chlorpheniramine maleate mixture was increased by microspheres of individual components. For isolation from tissues.e.g. KCl, aspirin. For administration in solid state and dry handling liquids such as eprazine can be converted to a pseudo solid by microspheres as an aid to handling and storage. It is used to mask bitter or unpleasant taste of the drug.e.g. Guanidine, clofibrate, paracetamolIt is used as an antidote in the poisoning of heavy metals.e.g. Polymercaptal microspheres on an antidote against mercury poisoning. To facilitatehandling of toxic materials.e.g. Microspheres have been used to decrease potential danger in handling toxic substances like pesticides, fertilizers and certain pharmaceuticals. It is used as diagnostically tool.e.g. Microencapsulated radios labeled molecules have been used for diagnostic testing of free drug or hormone concentrations, such as thyroxin estimation.
Several techniques are used for preparation of Microspheres like. 1.Polymer Phase Separation: Also referred to as coacervation.Polymer phase separation in non-aqueous media by non-solvents or polymer addition.2. Solvent evaporation & solvent extraction: The polymeric supporting material is dissolved in a volatile organic solvent; the active medicinal principle to be encapsulated is then dispersed or dissolved in organic solution to form a suspension, emulsion or solution. Then organic phase is emulsified under agitation in a dispersing phase consisting of a non-solvent of the polymer, which is immiscible with the organic solvent, which contains an appropriate surface active additive. Once the emulsion is 3.Stabilized, agitation is maintained & solvent evaporates after diffusing through the continues phase. The result is the creation of solid microspheres. On the completion of solid evaporation process, the microspheres held in the suspension in the continuous phase are recovered by filtration or centrifugal & washed & dry.
4. Wax Coating & hot-melt technique: Wax is used to coat the core particles.5. Spray coating & pan coating: Heat-Jacketed coating pan is used in which solid drug core particles are rotated & into which the coating materials is sprayed.6.PRECIPITATION7.Freeze-drying: Freezing of emulsion.8.Chemical & thermal cross-linking: Microspheres are made from natural polymers, which are prepared by a cross linking-process. The polymer includes gelatin, albumin, starch, and dextrin etc.
[adsense:468x15:2204050025]
Material:
Dichloromethane, methanol, ethyl cellulose, Tween-80, SLS, Michenical stirrer, filter paper etc.
Method: Prepare binary mixture (1:2) of dichloromethane & methanol. Add drug & ethyl cellulose (in 1:2 ratios) into it. Pour above mixture into the 200ml water previously added 0.05% of SLS then stir it with for at least 3 hr at 800-1200 RPM, allow stirring ,filter with filter paper ,then dry & microspheres are subjected to evaluate for further studies.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
|
INGRADIENTS |
F-1 |
F-2 |
F-3 |
|
EC(Ethyl Cellulose) |
2gm |
2gm |
2gm |
|
DS(Diclofenac Sodium) |
1gm |
1gm |
1gm |
|
Methanol: DCM |
1:1 |
1:4 |
1:4 |
|
Tween-80 |
0.5% |
0.5% |
- |
|
Water |
100ml |
100ml |
200ml |
|
SLS |
- |
- |
0.05% |
|
Spheres |
NOT FORMED |
NOT FORMED |
FORMED |
|
Evaluation parameters |
- |
- |
- |
|
Practical Yield |
- |
- |
1.22gm |
|
% Practical Yield |
- |
- |
41% |
|
Bulk Density |
- |
- |
0.58 |
|
True Density |
- |
- |
0.61 |
|
HausnerRatio (HR): |
- |
- |
1.05 |
|
Carr’s Index |
- |
- |
4.91 |
|
Angle of Repose |
- |
- |
22.78O |
Particle Size & Size Distribution:
|
PARTICLE No. |
EYE PIECE SIZE |
SIZE (u) |
|
1 |
16 |
213.28 |
|
2 |
15 |
199.95 |
|
3 |
11 |
146.63 |
|
4 |
18 |
239.94 |
|
5 |
19 |
253.27 |
|
6 |
14 |
186.62 |
|
7 |
17 |
226.61 |
|
8 |
18 |
239.94 |
|
9 |
18 |
239.94 |
|
10 |
19 |
253.27 |
|
Average size |
16.5 |
220 |
|
FREQUENCY(Nos) |
u |
PARTICLE SIZE |
|
7 |
173.29 |
13 |
|
10 |
186.62 |
14 |
|
18 |
199.95 |
15 |
|
10 |
213.88 |
16 |
|
11 |
226.61 |
17 |
|
13 |
239.94 |
18 |
|
10 |
253.27 |
19 |
|
21 |
266.6 |
20 |
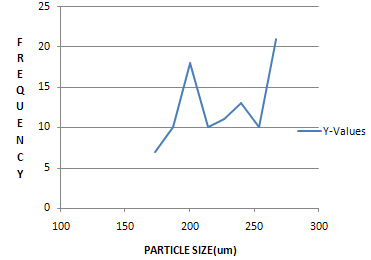
Figure: 1: Numbers of particles Vs Average diameter
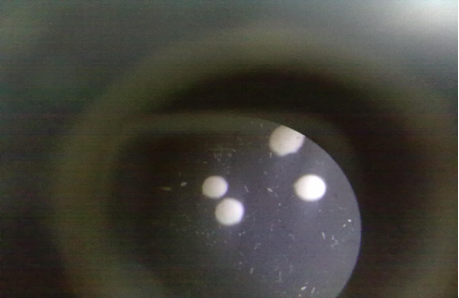
(Figure: 2: Photograph of Diclofenac Sodium Microspheres)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Drug content in Microspheres:
Au/As=Cu/Cs
Where,
Au=absorbance of microsphere solution=0.02
As=absorbance of drug (diclofenac) solution=0.566
Cu=concentration of drug (diclofenac) in solution of microsphere=?
Cs=concentration of drug (diclofenac) =10um/ml
0.02/0.566=Cu/10 so Cu=0.3534um
So 50 mg of microsphere of drug diclofenac contains
0.3534 X dilution factor (5000) =1767um=1.8mg
50 mg of microsphere of drug diclofenac contains 1.8gm of drug diclofenac
So 1220mg (Practical Yield) microsphere of drug diclofenac contains 1220X1.8/50 =43.92mg
1000mg diclofenac=100% so 43.92mg diclofenac
4.39%→DRUG CONTENT IN MICROSPHERE
Discussion: Microspheres are prepared by solvent evaporation method in which organic solvent (MeOH: DCM) ratios is taken 1:4.Tween-80 & SLS acts as a surface Active agent. Microspheres containing SLS having a spherical shape than Tween-80.rotation speed 500-600 RPM keep constant in all three formulas
Conclusion:
It could be concluded that the diclofenac: polymer ratio of 1:2 & organic solvent (MeOH: DCM) ratio of 1:4 was found to be optimum for spherical shape of microspheres as well as Practical Yield. Drug content found very low which could be due to high solubility of Diclofenac sodium in water which acts as an external phase so most of drugs goes into water during formulations of microspheres. Flow properties found was very good in case of Carr’s index, Angle of Repose while Hausner Ratio.
Acknowledgement: we are very much thankful to our head of Pharmaceutical Department & each & every person of our college who actually helpful to us in such type of research work.
References:
1. Laura J.Waters & Evangelos V.Pavlakis, In vitro controlled drug release from loaded microspheres-dose regulation through formulation, Journal of pharmaceutical science, 2007, 10, 4,464-472.
2. Vidyavathi Maravajhala,Nirmala Dasari,Asha Sepuri,S Joginapalli, Design & Evaluation of Niacin Microspheres-Indian journal of pharmaceutical sciences-2009;71;6;663-669.
3. Ajinath E Shirsat,Vaibhav V Gatade,Sanjeevani S Deshkar,Satish V.Shirolkar
4. Formulation & optimization of HPMC as MF microspheres using factorial design, Inventi Impact: pharmaceutical process development,2012 issue 2, (E-ISSN 2250-0340, P-ISSN 2249-3638)
5. S.P.Vyas &R.K.Khar, Targeted & Controlled Drug Delivery Novel carrier systems, “Microsphere” First edition Reprint: 2007, 417-457.
6. Raymond C.rowe, Paul J.Sheskey & Marian E.Quinin, A Handbook of Pharmaceutical Excipients, 6thEdition.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









