About Author: Vipul Kumar Tyagi (B.Pharm)
Shri Gopichand College Of Pharmacy,
Ahera (Baghpat)
Introduction
Tablet is the most popular among all dosage forms existing today because of its convenience of self administration, compactness and easy manufacturing; however hand tremors, dysphasia in case of geriatric patients, the underdeveloped muscular and nervous systems in young individuals and h case of uncooperative patients, the problem of swallowing is common phenomenon which leads to poor patient compliance. To overcome these drawbacks, mouth dissolving tablets (MDT) or orally disintegrating tablets; (ODT) has emerged as alternative oral dosage forms. These are novel types; of tablets that disintegrate/dissolve/ disperse in saliva within few seconds. A fast dissolving tablet was prepared by using various super disintegrants taken in different concentration (10%, 12%, and 14%) and one control batch is prepared without disintegrants designated as four different groups of formulations(F-1,F-2,F-3 and F-4) . Chemical incompatibility studies confirmed that there is no interaction between drug and excipients used in the formulations. All the batches are prepared by direct compression method. Effect of disintegrants on the disintegration behaviour was evaluated, and all the tablets were evaluated for hardness, friability, weight variation, water absorption ratio, dissolution, and assay.Direct compression method involves the incorporation of superdisintegrants in to the formulation. Direct compression does not require water or heat during formulation procedure and it is well suited for moisture and heat sensitive drugs. Fast dissolving tablets have so many advantages over liquid dosage form and conventional tablets. Fast dissolving tablet is suited for tablets which are undergoing first pass effect, and it is increase their bioavailability. A fast-dissolving drug delivery system, in most cases, is a tablet that dissolves or disintrigrants in the oral cavity without the need of water or chewing. Most fast-dissolving delivery system films must include substances to mask the taste of the active ingredient. This masked active ingredient is then swallowed by the patient’s saliva along with the soluble and insoluble excipients. These are also called melt-in-mouth tablets, repimelts, porous tablets, oro-dispersible, quick dissolving or rapid disintegrating tablets.
Reference ID: PHARMATUTOR-ART-1117
An ideal Properties of Fast Dissolving Tablets
· Require no water for oral administration, yet dissolve / disperse/ disintegrate in mouth in a matter of seconds.
· Have a pleasing mouth feel.
· Have an acceptable taste masking property.
· Be harder and less friable Leave minimal or no residue in mouth after administration
· Exhibit low sensitivity to environmental conditions (temperature and humidity).
· Allow the manufacture of tablet using conventional processing and packaging equipments.
Advantages of Fast Dissolving Tablets
Administration to the patients who can not swallow, such as the elderly, stroke victims, bedridden patients, patients affected by renal failure & patients who refuse to swallow such as pediatric, geriatric & psychiatric patients. Rapid drug therapy intervention. Achieve increased bioavailability/rapid absorption through
pregastric absorption of drugs from mouth, pharynx & oesophagus as saliva passes down. Convenient for administration and patient compliant for disabled, bedridden patients and for travelers and busy people, who do not always have access to water. Good mouth feel property helps to change the perception of medication as bitter pill particularly in pediatric patients. The risk of chocking or suffocation during oral administration of conventional formulations due to physical obstruction is avoided, thus providing improved safety. New business opportunity like product differentiation, product promotion, patent extension and life cycle management.
Salient Features of Fast Dissolving Drug Delivery System
· Ease of administration to patients who refuse to swallow a tablet, such as paediatric and geriatric patients and, psychiatric patients.
· Convenience of administration and accurate dosing as compared to liquids.
· No need of water to swallow the dosage from, which is highly convenient feature for patients who are traveling and do not have immediate access to water.
· Good mouth feels properly of MDDS helps to change the basic view of medication as “bitter pill”, particularly for paediatric patients.
· Rapid dissolution of drug and absorption which may produce rapid, onset of action.
· Some drugs are absorbed from the month pharynx and oesophagus as the saliva passes down into the stomach, in such cases bioavailability of drugs is increased.
· Ability to provide advantages of liquid medication in the form of solid preparation.
· Pregastric absorption can result in improved bioavailability and as a result of reduced dosage, improved clinical performance through a reduction of unwanted effects.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Conventional Techniques used for preparation of Fast Dissolving Drug Delivery System
Disintegrant Addition
Disintegrant addition technique is one popular technique for formulating Fast-dissolving tablets because of its easy implementation and cost-effectiveness. The basic principle involved in formulating Fast-dissolving tablets by disintegrant
addition technique is addition of superdisintegrants in optimum concentration so as to achieve rapid disintegration along with the good mouth feel. Microcrystalline cellulose and low substituted hydroxypropylcellulose were used as disintegrating agents in the range of 8:2 – 9.1 to prepare fast dissolving tablet. Agar powder is used as disintegrant for the development of rapidly disintegration tablets by enhancing the porosity of agar by water treatment. Rapidly disintegrating tablets of bitter drugs oxybutynin & pirenzepine were prepared by using the taste masked granules and h mixture of excipients consisting of crystalline cellulose (Avicel PH 02) and low-substituted hydroxypropy cellulose HPC, LH-11), Ishikawa et al. prepared rapidly disintegrating tablets using microcrystalline cellulose (Avicel PH-M series) that was spherical and had a very small particle size 7-32 ìm). instead of conventional microcrystalline cellulose (PH 102). Tablets prepared using microcrystalline cellulose; PH-M06 and L-HPC in the ratio of 9:1 were very rapidly disintegrating) in saliva. They concluded that Avicel PH-M06 was superior to Avicel PH 102 in terms of the feeling of roughness in the mouth. Fast dissolving table of efavirenz (anti HIV agent) were formulated by using combination of microcrystalline cellulose and sodium starch glycolate as super disintegrant. Gillis et al, prepared a fast-dissolving tablet of galanthamine hydrobromide which comprises of spray dried mixture of lactose monohydrate and microcrystalline cellulose (75:25) as a diluent, a cross linked polymeric disintegrant such as cross povidone and with a direct compression process of preparing such fast-dissolving tablets. Fast-dissolving tablets having analgesic activity was formulated using a combination of superdisintegrants. Rapid oral disintegration tablets were developed by direct compression using co-ground mixture of D-mannitol and crospovidone. CIMA labs patented Orasolv technology by employing the evolution of carbon dioxide or the effervescence as disintegration mechanism in the formulation of fast-dissolving tablets. The OraSolv technology is an oral dosage form, which combines taste-masked drug ingredients with a quick dissolving effervescent excipient system. Taste masking is achieved through a process of microencapsulation, which coats or entraps the active compound in an immediate release matrix. The effervescent excipient system aids in rapid disintegration of the tablet, permitting swallowing of pharmaceutical ingredients before they come in contact with the taste bud.
Freeze Drying
A process in which water is sublimated from the product after freezing. Lyophilization is a pharmaceutical technology which allows drying of heat sensitive drugs and biological at low temperature under conditions that allow removal of water by sublimation. Lyophilization results in preparations, which are highly porous, with a very high specific surface area, which dissolve rapidly and show improved absorption and bioavailability.
Moulding
In this method, molded tablets are prepared by using watersoluble ingredients so that the tablets dissolve completely and rapidly. The powder blend is moistened with a hydro-alcoholic solvent and is molded into tablets under pressure lower than that used in conventional tablet compression. The solvent is then removed by air-drying. Molded tablets are very less compact than compressed tablets. These possess porous structure that enhances dissolution.
Sublimation
The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa methelene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents.
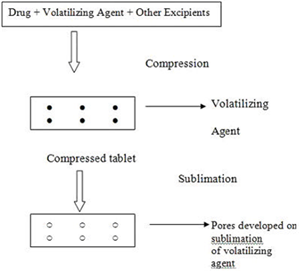
Steps Involved in sublimation
Spray-Drying
Spray drying can produce highly porous and fine powders that dissolve rapidly. The formulations are incorporated by hydrolyzed and non hydrolyzed gelatins as supporting agents, mannitol as bulking agent, sodium starch glycolate or crosscarmellose sodium as disintegrating and an acidic material (e.g. citric acid) and / or alkali material (e.g. I sodium bicarbonate) to enhance disintegration and dissolution. Tablet compressed from the spray dried powder disintegrated within 20 seconds when immersed in an aqueous medium.
Mass-Extrusion
This technology involves softening the active blend using the solvent mixture of water soluble polyethylene glycol, using methanol and expulsion of softened mass through the extruder or syringe to get a cylinder of the product into even segments using heated blade to form tablets. The dried cylinder can also be used to coat granules of bitter tasting drugs and thereby masking their bitter taste.
Direct Compression
It is the easiest way to manufacture tablets. Conventional equipment, commonly available excipients and a limited number of processing steps are involved in direct compression. Also high doses can be accommodated and final weight of tablet can easily exceed that of other production methods. Directly compressed tablet’s disintegration and solubilization depends on single or combined action of disintegrants, water soluble excipients and effervescent agent.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Patented Technologies For Fast Dissolving Tablets
Zydis Technology
Zydis, the best known of the fast-dissolving/disintegrating tablet preparations was the first marketed new technology tablet. The tablet dissolves in the mouth within seconds after placement on the tongue. A Zydis tablet is produced by lyophilizing or freeze-drying the drug in a matrix usually consisting of gelatin. The product is very lightweight and fragile, and must be dispensed in a special blister pack. Patients should be advised not to push the tablets through the foil film, but instead peel the film back to release the tablet. The Zydis product is made to dissolve on the tongue in 2 to 3 seconds. The Zydis formulation is also self-preserving because the final water concentration in the freeze-dried product is too low to allow for microbial growth.
Durasolv Technology
Durasolv is the patented technology of CIMA labs. The tablets made by this technology consist of a drug, fillers and a lubricant. Tablets are prepared by using conventional tableting equipment and have good rigidity. These can be packed into conventional packaging system like blisters. Durasolv is an appropriate technology for products requiring low amounts of active ingredients.
Orasolv Technology
Orasolv Technology has been developed by CIMA labs. In this system active medicament is taste masked. It also contains effervescent disintegrating agent. Tablets are made by direct compression technique at low compression force in order to minimize oral dissolution time. Conventional blenders and tablet machine is used to produce the tablets. The tablets produced are soft and friable and packaged in specially designed pick and place system.
Flash Dose Technology
Flash dose technology has been patented by Fuisz. Nurofen meltlet, a new form of ibuprofen as melt-in-mouth tablets, prepared using flash dose technology is the first commercial product launched by Biovail Corporation. Flash dose tablets consists of self binding shearform matrix termed as “floss”. Shearform matrices are prepared by flash heat processing.
Wowtab Technology
Wowtab Technology is patented by Yamanouchi Pharmaceutical Co. WOW means “Without Water “. In this process, combination of low mouldability saccharides and high mouldability saccharides is used to obtain a rapidly melting strong tablet. The active ingredient is mixed with a low mouldability saccharide and granulated with a high mouldability saccharide and compressed into tablet.
Flashtab Technology
Prographarm laboratories have patented the Flashtab technology. Tablets prepared by this system consist of an active ingredient in the form of micro crystals. Drug micro granules may be prepared by using the conventional techniques like coacervation, micro encapsulation, and extrusion spheronisation. All the processing utilized conventional tabletting technology.
Promising Drugs to be in corporated In Fast Dissolving Tablets
There are no particular limitations as long as it is a substance which is used as a pharmaceutical active ingredient.
Analgesics and Anti-inflammatory Agents:
Aloxiprin,
Auranofin,
Azapropazone,
Benorylate,
Diflunisal.
Anthelmintics:
Albendazole,
Bephenium Hydroxynaphthoate,
Cambendazole,
Dichlorophen,
Mebendazole,
Anti-Arrhythmic Agents:
Amiodarone,
Disopyramide,
Flecainide Acetate,
Quinidine
Sulphate.
Anti-bacterial Agents:
Benethamine Penicillin,
Cinoxacin,
Ciprofloxacin,
Clarithromycin,
Clofazimine,
Anti-coagulants:
Dicoumarol,
Dipyridamole,
Nicoumalone,
Phenindione.
Anti-Depressants:
Amoxapine,
Ciclazindol,
Maprotiline,
Mianserin,
Nortriptyline,
Anti-Epileptics:
Beclamide,
Carbamazepine,
Clonazepam,
Ethotoin,
Methoin,
Anti-Fungal Agents:
Amphotericin,
Butoconazole Nitrate,
Clotrimazole,
Econazole Nitrate,
Fluconazole,
Anti-Hypertensive Agents:
Amlodipine,
Carvedilol,
Benidipine,
Darodipine,
Dilitazem,
Anti-Malarials:
Amodiaquine,
Chloroquine,
Chlorproguanil,
Halofantrine,
Mefloquine,
Anti-Thyroid Agents:
Carbimazole,
Propylthiouracil.
Anxiolytic, Sedatives, Hypnotics And Neuroleptics:
Alprazolam,
Amyiobarbitone,
Barbitone,
Bentazeparn,
Bromazepam,
Diuretics:
Acetazolarnide,
Amiloride,
Bendrofluazide,
Bumetanide,
Chlorothiazide,
Gastro-Intestinal Agents:
Bisacodyi,
Cimetidine,
Cisapride,
Diphenoxylate,
Domperidone,
Opioid Analgesics:
Codeine,
Dextropropyoxyphene,
Diamorphine,
Dihydrocodeine,
Meptazinol,
Stimulants:
Amphetamine,
dexamphetamine,
dexfenfluramine,
fenfluramine,
mhazindol,
pemoline.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
METHODOLOGY-
1. SELECTION OF DRUG-
Diclofenac sodium is used to treat severe pain.It is also given in angina pectoris.It gives quick onset of action so,it is an important drug in present drug market.so, we selected diclofenac sodium for preparation and evaluation.
2. SELECTION OF EXCIPIENTS-
We used various excipients which are used in the preparation of drug.some lubricants such as Magnesium stearate and Talc are used in all four formulations.The other excipients such as MCC,Citric Acid,Menthol,SLS,Sodium CMC are used in different preparations to achieve desired properties of the preparation.
3. DRUG AUTHENTICATION-
A. Determination of melting point
B. Determination of IR Spectra
Determination of IR Spectra: ( by press pellet technique )
In this method a small amount of finely ground solid sample is intimately mixed with about 100 times its weight of powdered potassium bromide. The finely ground mixture is then passed very high pressure in a press (at 25,000 p sig) to form a small pellet (about 1-2 m thick and cm in diameter). The resulting pellet is transparent to IR radiation. Now this pellet is use to IR spectra with help of INFRA ABSORPTION SPECROSCOPY
Determination of melting point:-
Melting point is determine by theil’s tube method. In this method a small theil’s tube is tied with thermometer. Now put the sample, whose melting point is determine, in the theil’s tube .Dip the thermometer in liquid sample which have the high melting point. On the heating liquid sample, the temperature at which sample melt , taken as melting point.
4. INCOMPATIBILITY STUDIES-
Incompatibility study has been done by taking drug and excipient in ratio 1:1,and keep in oven. Change physically appearance of matrix tablet of mebendazole by keeping at 400 c for one month. There is no change physically appearance of matrix tablet
PREPARATION OF TABLET-
FORMULATION FOR ONE TABLET-
|
INGREDIENTS |
FORMOLA-1 |
FORMULA-2 |
FORMULA-3 |
FORMULA-4
|
|
DRUG(DICLOFENAC SODIUM) |
100mg. |
100mg. |
100mg. |
100mg. |
|
MCC |
100mg. |
100mg. |
100mg. |
20mg. |
|
CITRIC ACID |
50mg. |
30mg. |
30mg. |
30mg. |
|
MENTHOL |
- |
- |
20mg. |
- |
|
SLS |
- |
20mg. |
- |
|
|
SODIUM CMC |
- |
- |
- |
100mg. |
|
MAGNESIUM STEARATE |
1mg. |
1mg. |
1mg. |
1mg. |
|
TALC |
1mg |
1mg. |
1mg. |
1mg. |
BY DIRECT COMPRESSION METHOD-
PROCEDURE-
Direct compression method involves following steps
- Blending
- Compression
Blending Procedure For Preparation Of Mixed Blend Of Drug And Excipients
All ingredients were mixed as per the formulations.Super disintegrates were incorporated in the powder mix. And finally magnesium stearate and talc were added as lubricant. Control tablet was prepared without any super disintegrant.
Compression
MixedBlends were compressed by direct compression method using hand operated single punch machine.
Evaluation Test For Fast Dissolving Tablet-
Tablets from all the formulation were subjected to following
quality control test.
1. General Appearance
The general appearance of a tablet, its visual identity and over all “elegance” is essential for consumer acceptance. Include in are tablet’s size, shape, colour, presence or absence of an odour, taste, surface texture, physical flaws and consistency and legibility of any identifying marking.
2. Size and Shape
The size and shape of the tablet can be dimensionally described, monitored and controlled
3. Uniformity of weight
I.P. procedure for uniformity of weight was followed, twenty tablets were taken and their weight was determined individually and collectively on a digital weighing balance. The average weight of one tablet was determined from the collective weight. The weight variation test would be a satisfactory method of determining the drug content uniformity.
Sr. No. Average weight of Tablets(mg) Maximum percentage different allowed
1 130 or less 10
2 130-324 7.5
3 More than 324 5
Table: I.P. Specification for uniformity of weight
4.Tablet hardness
Hardness of tablet is defined as the force applied across the diameter of the tablet in the order to break the tablet. The resistance of the tablet to chipping, abrasion or breakage under condition of storage transformation and handling before usage depends on its hardness. Hardness of the tablet of each formulation was determined using Monsato Hardness tester.
5. Friability
It is measured of mechanical strength of tablets. Roche fribaiator was used to determine the friability by following procedure. A preweighed tablet was placed in the fribaiator. Fribaiator consist of a plastic-chamber that revolves at 25 rpm, dropping those tablets at a distance of 6 inches with each revolution. The tablets were rotated in the friabalator for at least 4 minutes. At the end of test tablets were dusied and reweighed, the loss in the weight of tablet is the measure of friability and is expressed in percentage as %Friability = loss in weight / Initial weight x 100
6. In Vivo Dsintegration test
The test was carried out on 6 tablets using the apparatus specified in I.P.-1996 distilled water at 37ºC ± 2ºC was used as a disintegration media and the time in second taken for complete disintegration of the tablet with no palable mass remaining in the apparatus was measured in seconds.
7. Wetting time
The wetting time of the tablets was measured using a simple procedure. Five circular tissue papers of 10cm diameter were placed in a Petri dish containing water (6ml). A tablet was carefully placed on the surface of tissue paper. The time required for water to reach the upper surface of the tablet was noted as the wetting time.
8. Dissolution Studies
Dissolution was carried out by using Electro lab dissolution apparatus (USP XXI) by paddle method using 900ml of 1%w/v SLS as the medium and rotating the paddle at 50 rpm for 10 minutes. The temperature of dissolution medium was maintained at 37±20C. Aliquots were withdrawn at different time intervals of 0, 2, 4, 6, 8 and 10 minutes. And it was replaced by adding equal volumes of fresh dissolution medium. The samples were suitably diluted and absorbance of the solution was determined at 368nm by using UV-visible spectrophotometer.
Drug release study was carried out by using specification given:-
1. Apparatus : Dissolution apparatus
2. Dissolution : 0.1 N Hcl
3. Temperature : 370 c
4. RPM : 50
5. Vol. withdraw & replace : 10 ml for every 30 min & later 1 hour for two sample 6. λ max : 368 nm
7. Blank solution : 0.1N Hcl
RESULT-
Data for Standard curve of Diclofenac sodium-
|
S.N. |
Concentration (µg/ml) |
Absorbance (nm) |
|
1 |
10 |
0.310 |
|
2 |
20 |
0.728 |
|
3 |
30 |
1.1 |
|
4 |
40 |
1.42 |
|
5 |
50 |
1.75 |
STANDARD CURVE OF DICLOFENAC SODIUM
IR Spectra-
I.R. spectra was obtained using KBr pellet method and compared with spectra as given in Indian Pharmacopoeia.
Melting point
|
Parameters |
Literature value |
Observed value |
|
Melting point |
283 – 285 |
280 – 287 |
Incompatibility studies–
EVALUATION-
|
PARAMETERS |
FORMULA-1 |
FORMULA-2 |
FORMULA-3 |
FORMULA-4 |
|
HARDNESS |
4.4 |
1.4 |
2.2 |
1.1 |
|
WEIGHT VARIATION |
1.28% |
3.5% |
4.55% |
2.62% |
|
FRIABILITY |
.95% |
0.90% |
0.82% |
0.75% |
|
WETTING TIME |
12.57 min. |
07.35 min. |
7.55 min |
6.42 min. |
|
DISINTEGRATION TEST |
8.57min. |
7.04 min. |
6.50 min. |
4.30 min. |
Data for Drug release study of formulations-
|
TIME INTERVAL(MINUTE) |
FORMULA-1 |
FORMULA-2 |
FORMULA-3 |
FORMULA-4 |
|
5 |
35 |
38 |
42 |
51 |
|
10 |
48 |
46 |
49 |
57 |
|
15 |
62 |
58 |
59 |
59 |
|
20 |
68 |
69 |
64 |
74 |
|
25 |
79 |
78 |
74 |
79 |
|
30 |
87 |
83 |
89 |
94 |
DISCUSSION
In formulation -1 we used MCC in the formulation of Diclofenac sodium.MCC is a surfactant which is used in the preparation of Diclofenac sodium tablets due to its surface enhancing property.But we could not get the desired tablet.
So, in formula -2 we used sodium lauryl sulphate with citric acid to enhance the dissolution of the tablet.In this formulation we get the less disintegration time than formula-1.In formula-3 we used menthol which is a volatile substance.after keeping tablet in oven at 50’ c for 1 hour.after drying the menthol evaporated and left small pores on the surface of the tablet which enhance the dissolution and disintegration.In formula-4 CMC is used in the formulation because of its highly hydrophilic nature which enhance dissolution and disintegration and we get the desired formulation.
References
1. Lieberman H. A., Lachman L. & Kanig L. J. Theory & Practice of Industrial Pharmacy, 3rd Edition, Varghese Publishing House, Mumbai, 2008; 412-428.
2. Tripathi K.D. Essentials of Medical Pharmacology, 6th Edition, Jay Pee Publishers, New Delhi, 2008; 193.
3. Indian Pharmacopoeia, Vol.-I, The Controller of Publication, Delhi, 1996; 135.
4. Jain N.K. Advance in Controlled & Novel Drug Delivery System, 4th Edition, Vallabh Prakashan, New Delhi, pg. No. 3-4 & 107
5. Vyas SP, Khar RK. Controlled drug delivery: Concepts an advances:drug delivery; p.411-476.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









