ABOUT AUTHOR:
BINDU.S
ANNAMACHRYA COLLEGE OF PHARMACY,
RAJAMPET,KADAPA Dist, A.P, INDIA.
sirasalabindupharmacy@gmail.com
INTRODUCTION
Rock salt, also known as halite is the naturally occurring crystalline salt. It is the mineral form of sodium chloride or the table salt that we use. However, rock salt does not look like the regular table salt. Instead, it naturally forms in large isometric crystals. Moreover, rock salt can be white like table salt or it can be red, orange, yellow or blue in color. The color of rock salt basically depends on the amount, and the kind of impurities present in it. Along with sodium, rock salt contains many other minerals including, calcium, zinc, iron, magnesium, potassium, copper and several other trace minerals. Like table salt, rock salt too has great many uses. rock salt in massive bags for the purpose of keeping down ice on the roads in the winter.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1707
Formation
Halite forms when seawater dries up, leaving the mineral deposits behind. The evaporated seawater leaves behind positive sodium and negative chlorine ions .Rock salt forms naturally, when the saline water of sea evaporates. It is mined from underground deposits, which form when the inland seas evaporated millions of years ago. Such rock salt deposits can be found in inland marginal seas, dry lake beds, as well as enclosed bays and estuaries, found in certain arid regions
Chemical constituents
It is mainly composed of 78.84% of sodium chloride and 18.92% of water insoluble materials like quartz, clay, mud and marl it also minerals like sodium, potassium, magnesium, calcium, phosphorus, iron, zinc iodide.
Uses
Rock salt is used in making ice creams. However, it is not a part of the ice creammixture. Instead, it is used to melt the ice that surrounds the can. When packed in with ice in a ice cream maker, rock salt can lower the temperature of the ice cream maker, so that ice cream can get even colder.rock salt is used as food preservative. Rock salt is used to make lamps. These lamps produce negative ions, which in turn, attach to pollens and other environmental allergens, which helps to purify air..Rock salt is employed for softening hard water.Rock salt acts as a fixing agent while dyeing clothes, which is an important industrial use of this salt.Apart from these, rock salt is used by many to treat many types of skin disorders, pain, inflammation, cold and cough and the pain caused by rheumatism.
Lemon
The lemon is both a small evergreen tree (citrus -limon, often given as C. limon) native to Asia, and the tree's ellipsoidal yellow fruit. The fruit is used for culinary and non-culinary purposes throughout the world – primarily for its juice, though the pulp and rind are also used, mainly in cooking and baking. Lemon juice is about 5% to 6% (approximately 0.3 M) citric acid, which gives lemons a sour taste, and a pH of 2–3. Many lemon flavored drinks and foods are available, including lemonade and sherbet lemons, as well as lemon and seasoning salt as a snack. The distinctive sour taste of lemon juice makes it a key ingredient in many dishes across the world.
Pharmacological uses
Culinary uses
Lemon juice is used to make lemonade, soft drinks, cocktails, and marinades.used as a short-term preservative.Lemon slices and lemon rind are used a garnish for both food and drinks..Lemon zest, the grated outer rind of the fruit, is used to add flavor to baked goods, puddings, rice and other dishes
Non culinary uses
Aromatherapy, first aid and medicine.Lemon oil aroma used in aromatherapy may enhance mood. The low pH of juice makes it antibacterial
Insecticide
The d-limonene in lemon oil is used as a nontoxic insecticide treatment.
[adsense:468x15:2204050025]
AIM AND PLAN OF WORK
Rock salt is one of the most well know substance and it has many properties. Upon from this it is used to treat skin disorders, pain, inflammation cold and cuff citric acid is one of the major constituents in lemon approximately 5-6% present as a ph of 2-3 it is used as a short term preservatives ,as a flavoring agent as backed good, pudding, rice, other dishes
On the basis of literature survey various journal has been made to study about the current status of selected materials even both the materials are well known in Indian traditional medicine and having many medicinal properties no scientific data have been published so far supporting the combination identification, characterization, and medicinal properties
Thus this study was aimed to access the characterization, identification of constituents through IR, NMR, and MASS spectroscopy studies further the identification constituents are
Also proposed to find the possible biological activities.
Plan of work:
Collection of rock salt and lemonand Extraction of lemon juice and this is Characterization by FTIR and there is a Individually characterization of rock salt and citric acid.
MATERILES AND METHODS
List of chemicals
Sodium hydroxideSulphuric acidFerric chloridePyridineCPotassium bromide alcium hydroxidePotassium bromide
instruments
FTIR-01517IR Affinity-1
List of methods
Isolation of citric acid
Identification of citric acid
Sample preparation containing rock salt and citric acid
characterization of rock salt, citric acid, and sample
EXPERIMENTAL INVESTIGATION
Lemon weresqueezed and the juice obtained was filtered through the Buchner funnel. To the about 50 ml of lemon juice add 15%NAOH with constant stirring until the mixture is slightly alkaline as indicated by colour changes from clear yellow to brown.
To the filtrate add 5ml of 10% calcium chloride solution for each 10ml of filtrate, heat to boiling and filter off while hot cuprous precipitate of calcium citrate through buechner funnel wash the precipitate with small volume of boiling water.
Resuspend the precipitate in minimum quantity of cold water, heat to boiling filter and allow the salt to air. Calculate the yield on dry weight basis’
Transformation of calcium citrate to citric acid
Ca3Cl2H10O14 +3H2SO4 → 2H3C6H5O7+3CaSo4
To transform calcium salt in to citric acid add to it the proper amount of 2m aqueous H2SO4 (CALCULATE IT ACCORDING TO THE REACTION). Stir with glass rod and leave to stand by for 10min. then filtrate the sediment of CaSo4 on a buechner funnel, move to the beaker and concentrate the aqueous layer to 10ml by boiling on a hot-plate Cool down concentrated soluction and after a few minutes the crystals of citric acid should appear. Filtrate the product obtained, dry it in the air and weight.
Measure the melting point (152-1540c) calcualate the yield of citric acid relative to lemon juice used.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CHARACTERIZATION BY FTIR:
Recently, spectroscopy has emerged as one of the major tool for natural dyes applications and has made significant progress in the field of clinical enaluation. FTIR spectrum is important to record it gives sufficient information about structure of compound. The absorction of IR radiation causes various band in a molecular to stretch and bend with respect to one another. The most important region the organic chemist is 2.5micron-15micron the region from 0.8-2.5 micron called near IR. And region from 15-200micron called far IR these bands are relatively narrow, easy to resolve, and sensitive to molecular structure, conformation, and environment.
Sample preparation
Mix the sample and kbr in ratio of 1:100 then grind mixture in to fine powder by using mortar and pestle then prepare the disc by introduction the mixture in to kbr press. The disc obtained is scanned in FTIR FROM 500-4000CM-1 three samples were analysed.citric acid, rock salt, rock salt and citric acid sample.
RESULTS AND DISCUSSION
Citric acid was isolated from the lemon juice and this is used for preparation of sample by mixing with rock salt
Citric acid isolated was identified various chemical tests
|
Test |
Observation |
Inference |
|
Test for acidity |
Highly acdic |
Citric acid is present |
|
Test for Fecl2 |
Yellow colour developed |
Citric acid is present |
|
Reaction with (CH3CO)2CO |
Red colour developed |
Citric acid is present |
|
Reaction with calcium chloride |
White precipitate is formed |
Citric acid is present |
Citric acid, rock salt, and sample containing rock salt and citric acid are characterized by FTIR
IR spectrum of rock salt
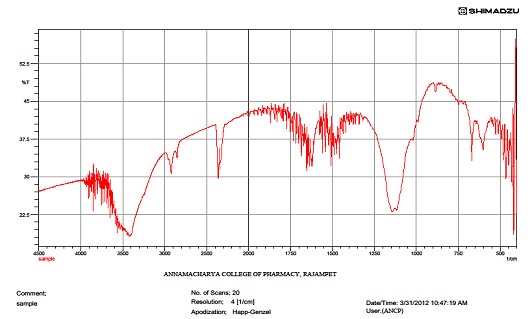
|
S.NO |
WAVE NUMBER IN CM-1 |
FUNCTIONAL GROUP |
|
|
1 |
3446 |
O-H str |
|
|
2 |
2845 |
C-H str |
|
|
3 |
1734 |
C=O str |
|
|
4 |
1224 |
C-C str |
|
IR spectrum of citric acid
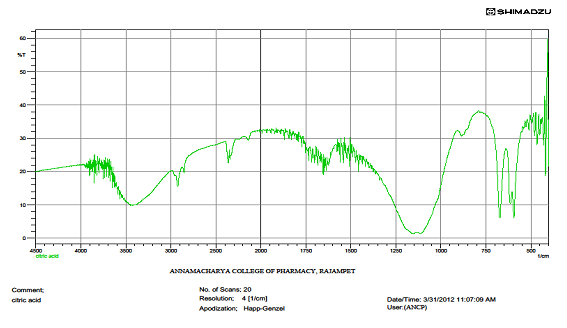
|
S.NO. |
WAVENUMBER IN CM-1 |
FUNCTIONAL GROUP |
|
1 |
3477 |
O-H str |
|
2 |
1707 |
C=O str |
|
3 |
1292 |
C-O def |
|
4 |
1386 |
C-O-H in plane bend |
|
5 |
933 |
O-H def |
|
6 |
700 |
C-H def |
IR spectrum of sample
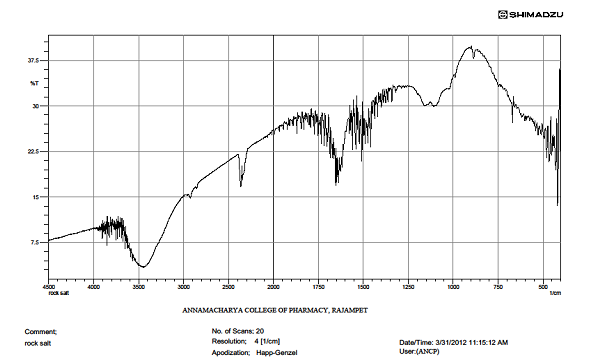
|
S.NO |
WAVE NUMBER IN CM-1 |
FUNCTIOMAL GROUP |
|
1 |
3500-3700 |
O-H str |
|
2 |
2545 |
C-H str |
|
3 |
1734 |
C=O |
|
4 |
1558 |
C-O |
|
5 |
1398 |
CH2 |
Characterized of Rock salt, citric acid, and sample by FTIR at 100
CONCLUSION
Citric acid was isolated and mixed with rock salt to preparethe sample and this rock salt citric acid and sample was characterization by FTIR at the conduction of the10o.
Further the sample will be analyse at different conditions and through NMR and MASS for investigational studies.
REFERENCES
1. pennistonKL, Nakada SY, Holmes RP, AssimosDG. Quantitative Assesment of citric acid in lemon juice, lime juice, and commercially-Available fruit juice products journal of Endourology(2008)22 (3):567-70.
2. Hu, Y.;-Y.; Rawal,A.;Schmidt-rohr.K. Strongly bound citrate stablilizes the apatite nanocrystals in bone. Proceedings OF THE NATIONAL academy of sciences 107:22425.
3. Tissues that fight germs. CNN.2004-07-14. Retrieved 2008-05-08.
4. Wright, A.clifford. History of Lemonade, CliffordAWright.com
5. Glusen, O.; M. L.Roose (2001). “lemons:Diversity and relationships with selected citrus genotypes as Measured with Nuclear Genome Markers”. Journal of the American society of Horticultural science, 126:309-317.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









