ABOUT AUTHORS:
Prajjval Makhaik
School Of Pharmaceutical Sciences,
Bahra University, Shimla Hills
prajjvalkotgarh777@gmail.com
ABSTRACT
Scaffolds represent important components for tissue engineering. Scaffolds are used for drug delivery in tissue engineering as this system is a highly porous structure that allows tissue growth. A scaffold provides a suitable substrate for cell attachment, cell proliferation, differentiated function, and cell migration. Scaffold matrices can be used to achieve drug delivery with high loading and efficiency to specific sites. Biomaterials used for fabrication of scaffold may be natural polymers such as alginate, proteins, collagens, gelatin, fibrins, and albumin, or synthetic polymers such as polyvinyl alcohol and polyglycolide. Bio ceramics such as hydroxyapatites and tricalcium phosphates also are used. Techniques used for fabrication of a scaffold include particulate leaching, freeze-drying, supercritical fluid technology, thermally induced phase separation, rapid prototyping, powder compaction, sol-gel, and melt moulding. These techniques allow the preparation of porous structures with regular porosity. Scaffold are used successfully in various fields of tissue engineering such as bone formation, periodontal regeneration, repair of nasal and auricular malformations, cartilage development, as artificial corneas, as heart valves, in tendon repair ,in ligament replacement, and in tumours. Scaffold also can be used to provide adequate signals (e.g., through the use of adhesion peptides and growth factors) to the cells, to induce and maintain them in their desired differentiation stage, and to maintain their survival and growth. Some of the important issues regarding scaffolds as drug delivery reviewed in this article.
Reference Id: PHARMATUTOR-ART-1983
INTRODUCTION
Tissue engineering aims to replace or facilitate the growth of damaged or diseased tissue by applying combinations of biomaterials, cells and bioactive molecules. Certain tissues in the body contain cells capable of initiating, regeneration or repair after injury. Tissue undergoing constant renewing e.g. Skin, bone marrow, intestinal mucosa is capable of complete regrowth. In comparison to other tissue types such as heart muscles and nerves lacks the mechanism of regeneration in adults .For these tissue type, stem cell biology offers the potential to grow tissue by following a delivery pathway and that is, scaffolds. In the last two decades, the research and development among the scientific community in this emerging field of tissue engineering and regenerative medicine has progressed at a rapid rate.(1) Biodegradable polymeric scaffolds for tissue engineering have received much attention because they provide a temporal and spatial environment for cellular growth and tissue in-growth.(2–4) Scaffold is the central component that is used to deliver cells, drugs, and genes into the body.
The definition of the scaffold is categorized into 2 main categories:
(1) A cell delivery scaffold and
(2) A drug delivery scaffold.
When cells are implanted or seeded into an artificial structure capable of supporting three-dimensional (3D) tissue formation, these structures typically are called “cell delivery scaffolds,” and when drugs are loaded into a 3D artificial porous structure capable of high drug loading efficiency and sustained release of a drug for longer duration, they typically are called “drug delivery scaffolds.’’(5)
Scaffold for tissue engineering (cell delivery) should posses the following properties:-
1. Mechanical properties that is sufficient to shield cells from tensile forces without Inhibiting biomechanical cues
2. Desired volume, shape, and mechanical strength.(4)
3. Acceptable biocompatibility
4. A highly porous and well-interconnected open pore structure to allow high cell seeding density and tissue in-growth
5. Bio adsorption at predetermined time period
6. Biocompatible chemical compositions and their degradation products, causing minimal immune or inflammatory response.( 9)
7. Physical structure to support cell adhesion and proliferation, facilitating cell– cell contact and cell migration(10)
Different forms of polymeric scaffolds for cell/drug delivery are available:
(1) Highly porous well interconnected open pore structure
(2) a nanofibrous matrix that is prepared by electro spinning
(3) a thermo sensitive sol-gel transition hydro gel
(4) a porous microsphere .
These are already widely utilized as sustained protein-release formulations and have been applied in tissue engineering for the potential use as a cell delivery carrier or supportive matrix.(6,7) Of the polymeric scaffolds listed above, a typical 3D porous matrix and nanofibrous matrix are the implantable forms and a thermo sensitive sol-gel transition hydro gel and porous microsphere are the injectable forms.
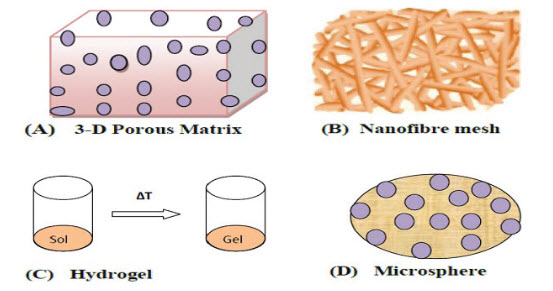
Figure 1. Matrix (A); nanofiber mesh (B); and Different forms of polymeric scaffolds for cell/drug/gene delivery: three-dimensional porous microsphere (D) hydro gel(C)
Tissue engineering technologies are based on this biological triad and involve the successful interaction between three components:
(1) The scaffold that holds the cells together to create the tissue’s physical form;
(2) The cells that create the tissue; and
(3) The biological signalling molecules, such as growth factors, that direct the cells to express the desired tissue phenotype .(9)
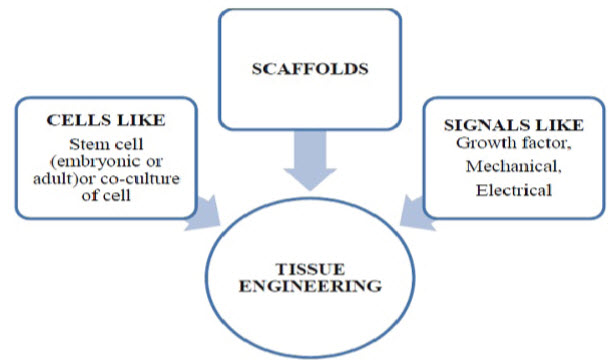
Figure 2. The tissue engineering triad
DESIGN STRATEGIES FOR CELL AND DRUG DELIVERY SYSTEMS
Although prefabricated scaffolds are most widely used for tissue regeneration as well as drug delivery purposes, different forms of polymeric scaffolds for cell/drug delivery are also available. These forms can be classified as (1) a typical 3D porous matrix, (2) a nanofibrous matrix, (3) a thermo sensitive sol-gel transition hydro gel, and (4) a porous microsphere. Of these, the typical 3D porous matrix and nano fibrous matrix are the implantable forms and the thermo sensitive sol-gel transition hydro gel and the porous microsphere are the injectable forms. Injectable scaffold materials formed in situ have received much attention recently because they can be administered using a syringe needle10 and thus avoid surgery. To mimic the topological and micro structural characteristics of the ECM, a biomaterial must have a high degree of porosity, a high surface: volume ratio, a high degree of pore interconnection, appropriate pore size, and geometry control.(11) These properties can be well controlled in an injectable scaffold. Some of the drug/cell delivery systems and their design strategies are given in the following sections:-
A.)Hydro gel-Based System
Hydro gel matrices are physically or chemically cross-linked, water-soluble polymers, which swell to form a gel like substance on exposure to water.(12) Hydro gels are appealing for biological applications because of their high water content and biocompatibility.(13) Hydro gels can be made from naturally occurring polymers such as collagen, chitosan, and gelatine or synthetic polymers such as poly(ethylene glycolide) and poly vinyl alcohol. Growth factors are released from hydrogels through diffusion of the growth factor A Novel Carrier for Cell and Drug Delivery(3) through the highly hydrophilic scaffold, mechanical stimulation, or hydrolytic degradation of the scaffold(12) or upon swelling in response to an environmental stimulus. For example, gelatin and dextran can be fabricated as an interpenetrating polymer hydro gel for drug delivery and can exhibit an intelligent property of degradation in response to dual stimuli.(14) Release behaviour can be regulated by controlling the chemical and physical properties of the gels from a few days to several months.(15) Above critical concentrations, these hydro gels show a sol state at room temperature, but change into a gel state at body temperature(15); hydro gels can be administered in a minimally invasive manner and therefore they are used in tissue engineering strategies as a potential cell and protein delivery vehicle.(11) Additional advantages of hydro gels are that they may protect drugs, peptides, and especially proteins against the potentially harsh environment in the vicinity of the release site; they enable enhanced residence times, sustained delivery, and/or targeted drug delivery (13); and they have significant potential in wound healing applications, though pore size and degradation properties must be optimized.(11) For example, injectable poly(N-isopropylacrylamide) physical hydro gels encapsulating cells have been prepared for cartilage and nerve regeneration.(16,17) Pluronic/heparin composite hydro gels delivering growth factor also have been studied to induce angiogenesis.(18) Photo crosslinked poly(ethylene glycol) (PEG)–based hydrogels have been utilized for delivery of chondrocytes and osteoblasts.(19–21) Bone morphogenic protein introduced into the hydrogel material (temperature-sensitive chitosan-polyol salt combination) has been effective in promoting de novo bone and cartilage formation in vivo.(22) Poly(lactic acid–glycolic acid) (PLGA) grafted with PEG and PEG grafted with PLGA hydrogels capable of sustained insulin delivery and cartilage repair were synthesized.(23) Pluronic copolymers at a higher concentration (more than 20% [w/v]) have been used to encapsulate chondrocytes and produce engineered cartilage.(24)
B.)Microsphere- and Microparticle-based Systems
Delivery has several potential advantages, such as the inherent stability of plasmid DNA, reduced fabrication costs, extended shelf-life, a more economical use , and application in skin repair.(26) Application is pellets incorporated with basic fibroblast growth factor– loaded microspheres into alginate porous scaffolds to enhance vascularization after implantation in the rat peritoneum. Chitosan scaffolds loaded with basic fibroblast Microspheres and micro particles have attracted attention as carrier matrices in both the biomedicine and bioengineering fields and could satisfy the need of delivering biomolecules such as growth factors, genes, and cells.(25) Prior to injection, the porous structure (30 μm) would allow sufficient cell seeding in and out of the matrix. After injection in vivo, the porous matrix would permit infiltration of cells and in growth of tissue from the host, facilitating the regeneration process.(15) Micro particles also can be used as injectable scaffolds to support cell growth and proliferation directly and as vehicles of growth factor, and to enhance cell proliferation and expansion simultaneously.(11) Microsphere based technology has an application for tissue engineering as well as gene therapy. Gene growth factor contained in gelatin microparticles were effective in accelerating wound closureCritical Reviews™ in Therapeutic Drug Carrier System Garg et al.of pressure ulcers.(27) Biodegradable PLGA microspheres have been studied for deliveryof chondrocytes for cartilage engineering.(10) Nanofabricated particles could offer betterdelivery properties to direct cell fate and to regulate processes such an angiogenesis andcell migration.(11)
C.)Membrane-based Systems
Human skin is considered the gold standard for treatment of skin wounds. However, skin grafts are not always the perfect solution. They are limited in terms of the conditions needed for tissue availability, graft rejections, and conformability with the surrounding tissue with respect to thickness and pigmentation.(27, 29) Current strategies for wound dressings have been aimed at the development of the bilayer-structured membrane, with incorporation of growth factors into these matrices for improved healing. For example, gelatin hydrogel containing epidermal growth factor–loaded microspheres has an enhanced effect on re-epithelization, improving the healing of the wound area. Antibiotics should be incorporated into the membranes to prevent infections because sustaining a sufficient drug concentration at the site of infection is important for the treatment of an infected wound. For example, a bilayered membrane combines silver sulphadiazine and a laminin-modified collagen membrane, which was shown to facilitate the dermal wound healing process.
Materials used for Scaffold preparation
Several natural and synthetic polymers have been utilized for fabricating tissue engineering scaffolds. The ideal polymers should be
1. Biocompatible
2. Biodegradable
3. Highly Cell adhesive
4. Porous , mechanically stable 3-D structure
Materials used for scaffold preparation of bone and cartilage
Hydroxyapatite(HA)
Poly (α-hudroxyesters)
Natural polymers such as collagen and chitin.
Polylactides(PLLA,PDLA)
Polycaprolactone(PCA)
A number of non-approved polymers, such as polyorthoester (POE) Polyanhydride are also available.
Biomaterials for Scaffolds formation
A number of different categories of biomaterials are commonly used as scaffold for cell and drug delivery.
A. Natural Polymers
Natural polymers include alginate, proteins, collagens, gelatin, fibrins, albumin, elsinan, pectin (pectinic acid), galactan, curdlan, gellan, levan, emulsan, dextran, pullulan, gluten, elastin, fibroin, hyarulonic acid, cellulose, starch, chitosan (chitin), scleroglucan, heparin, silk, chondroitin 6-sulfate, and polyhydroxyalkanoates. They can be used as biomaterials for cell/drug/gene delivery purposes. Advantages of natural polymers include their biocompatibility, commercial availability, easy processing, and they more closely mimic the natural ECM of tissues; however, limitations are short supply, expense, batch-to batch variation, and susceptibility to cross-contamination.(31)
B. Synthetic Polymers
Synthetic polymers are largely divided into two categories: biodegradable and nonbiodegradeable. Biodegradable polymers are polyglycolide, polylactide and its copolymer Poly (lactide-co-glycolide), polyphosphazene, polyanhydride.
Methods of preparation of scaffold
Various methods are used for preparation of 3-D porous scaffolds
1. Solvent casting/particulate leaching,
2. Emulsion freeze drying,
3. Rapid prototyping,
4. Thermally induced phase separation,
5. Gas foaming/particulate leaching,
6. High pressure processing,
1. Solvent casting/particulate leaching
Solvent casting/particulate leaching method is the most convenient method for preparing porous scaffolds. It involves the casting of polymer/salt/organic solvent mixture solution followed by solvent evaporation and dissolution of the salt particulates in aqueous solution.(49)
2. Emulsion freeze drying
Emulsion freeze drying method emulsion is prepared by dispersing water phase in organic continuous phase containing biodegradable polymer. This can give rise to porous scaffolds with various pore sizes.(50)
3. Rapid prototyping
Rapid prototyping (RP), also known as solid free from fabrication has recently introduced a new method in fabricating well-designed tissue engineering scaffolds.(51)
4. Thermally induced phase separation
The phase separation technique is based on thermodynamic demixing of homogeneous polymer solvent solution in to polymer poor phase, exposure of the solution to another immiscible solvent. Particularly thermally induced phase separation (TIPS) uses thermally induced phase separation .The polymer solution is quenched below the freezing point of the solvent and subsequently freeze dried, producing a porous structure.(52)
5. Gas foaming/ particulate leaching
In gas foaming/particulate leaching method effervescent salt is used as gas foaming agent. Binary mixture of polylactide(PLA) solvent gel containing dispersed ammonium bicarbonate particulate was cast in mold and subsequently immersed in hot water. The evolution of ammonia and carbon dioxide gas, along with leaching out of ammonium bicarbonate particulates from the solidifying polymer matrix, resulted in the formation of pores with high interconnectivities.(53)
6. High pressure processing
High pressure processing is also known as the supercritical fluid technology. It is performed by applying the gas such as cabon-dioxide to a dry polymer at high pressure, which forms single phase polymer gas solution. The pressure is then reduced to create thermodynamic instability of the dissolved carbon dioxide and result in nucleation and growth of gas cells to generate pores within polymer matrix.(54)
Use of Ceramics for the preparation of bone matrices
Ceramics composed of calcium phosphate, silica; alumina, zirconium and titanium dioxide are nowadays used for various medical applications due to their positive interactions with human tissues. Example: dentistry
These ceramic materials possess high mechanical strength, good body-response and low biodegradability. Due to slow degradability ceramic materials are more favoured in bone scaffolds technique. Especially hydroxyapatite based calcium phosphate compounds and bioactive glass are regarded as high potentials scaffolds due to their osteo-conductive properties.
CONCLUSION AND SUMMARY
Scaffold drug delivery system is the most interesting drug delivery system. Scaffold drug delivery is the most advantageous drug delivery systems as it facilitate the growth of damaged or diseased tissue by applying combination of biomaterials, cells and bioactive molecules. Scaffold drug delivery based on the use of biodegradable materials. By using the biodegradable material tissues or organs are prepared by simply implantation of the scaffolds are used. These include treating wound repair, tissue generation, bone and cartilage regeneration, neural tissue engineering and for delivering DNA .However, there is much work that needs to be carefully demonstrated for the scaffold drug delivery.
REFERENCE
1).Kretlow JD, Mikos AG. From material to tissue: biomaterial development, scaffold Fabrication and tissue engineering. AIChE J. 2008; 54(12):3048-67.
2.) Langer R, Vacanti JP. Tissue engineering. Science. 1993; 260:920–6.
3.) Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Nat Biotechnol. 1994; 12:689–93.
4.) Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000; 21:2529–43.
5.) Papkov MS, Agashi K, Olaye A, shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007; 59:187–206.
6.) Hoffman AS. Hydro gels for biomedical applications. Adv Drug Deliv Rev. 2002; 43:3–12
7.) Drury JL, Mooney DJ. Hydro gels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003; 24:4337–51.
8.) Lyons F, Partap S, O’Brien FJ. Part 1: scaffolds and surfaces. Technol Health Care. 2008; 16:305–17.
9.) Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. J Biomed Mater Res. 1998; 43:422–7.
10.) Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Delivery Rev. 2007; 59:249–59.
11.) Huang S, Fu X. naturally derived materials-based cell and drug delivery systems in skin regeneration. J Control Release. 2010; 142:149–59.
12.) Drury JL, Mooney DJ. Hydro gels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003; 24:4337–51.
13.) Hoffman AS. Hydro gels for biomedical applications. Adv Drug Delivery Rev. 2002; 43:3-12.
14.) Kurisawa M, Yui N. Gelatin/dextran intelligent hydro gels for drug delivery: dualstimuli-responsive degradation in relation to miscibility in interpenetrating polymer networks. J Control Release. 1998; 199:1547–54.
15.) Augst AD, Kong HJ, Mooney DJ. Alginate hydro gels as biomaterials. Macro mol Biosci. 2006; 6:623–33.
16.) Stiles RA, Burghardt WR, Healy KE. Synthesis and characterization of injectable poly (N-isopropylacrylamide)- based hydro gels that support tissue formation in vitro. Macromolecules. 1999; 32:7370–9.50 Garg et al.
17.) Park KH, Yun K. Immobilization of Arg-Gly-Asp (RGD) sequence in a thermo-sensitive hydrogel for cell delivery using pheochromocytoma cells (PC12). J Biosci Bioeng. 2004; 97:374–7.
18.) Yoon JJ, Chung H, Park TG. Photo-crosslinkable and biodegradable Pluronic/heparin hydrogels for local and sustained delivery of angiogenic growth factor. J Biomed Mater Res. 2006; 79:934–42.
19.) Bryant SJ. Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res. 2003; 64:70-9.
20.) Burdicka JA, Ansetha KS. Photo encapsulation of osteoblasts in injectable RGD-modified PEG hydro gels for bone tissue engineering. Biomaterials. 2002; 23:4315–23.
21.) Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrowderived mesenchymal stem cells in a photopolymerizing hydro gel. Tissue Eng. 2003; 9:679–88.
22.) Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, Leroux JC, Atkinson BL, Binette F, Selmani A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000; 21:2155–61.
23.) Jeong B, Lee KM, Gutowska A, an YH. Thermo gelling biodegradable copolymer aqueous solutions for injectable protein delivery and tissue engineering. Biomacromolecules. 2002; 3:865–8.
24.) Saim AB, Cao Y, Weng Y, Chang C, Vacanti MA, Vacanti CA, Eavey RD. Engineering autogenous cartilage in the shape of a helix using an injectable hydrogel scaffold. Laryngoscope. 2000; 110:1694–7.
25.) Tabata Y, Hijikata S, Muniruzzaman M, Ikada Y. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed. 1999; 10:79–94.
26.) Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003; 55:329–47.
27.) Park CJ, Clark SG, Lichtensteiger CA, Jamison RD, Johnson AJ. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater. 2009;5:1926–36.
28.) Clark RAF, Singer AJ. Wound repair: basic biology to tissue engineering. London: Academic Press; 2000.
29.) Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D. Systematic reviews of wound care management: (2). Dressings and topical agents used in the healing of chronic wounds. Health Technol Assess. 1999; 3:1–35.
30.) Lee JE, Park JC, Lee HK, Oh SH, Suh H. Laminin modified infection-preventing collagen membrane containing silver sulfadiazine–hyaluronan microparticles. Artif Organs. 2002; 26:521–8.
31.) Saltzman WM, Baldwin SP. Materials for protein delivery in tissue engineering. Adv Drug Delivery Rev. 1998; 33:71–86.
32.) Whang K. Engineering bone regeneration with bio absorbable scaffolds with novel micro architecture. Tissue Eng. 1999; 5:35–51.
33.) Mikos AG, Sarakinos G. Leite SM, Vacanti JP.Lang R. Laminated three-dimensional biodegradable foams for use in tissue engineering, Biomaterials 14:323-330(1993)
34.) Whag K, Thomas CH,Healy KE,A novel method to fabricate bioabsorbable scaffolds, polymer 36:837-842(1995)
35.) Yang S Leong K .DuZ. Chua C.The design of scaffolds for use in tissue engineering. Part 2. Rapid prototyping technique tissue engineering 8: 1-11(2002)
36.) Nam YS, Park TG, Biodegradable polymeric microcellular foams by modified thermally induced phase separation methods Biomaterials 20:1783-1790(1999)
37.) Nam YS Yoon JJ Park TG A novel fabrication method macroporous scaffolds using gas foaming salt as porogen additive ,J. Biomed Mater .Res (Appl. Biometer)53:1-7,(2000)
38.) Mooney DJ, Baldwin DF, Suh NP, Vacanti JP,Lang Langer R. NovelApproach to fabricate porous sponges of poly (D,L-lact-co glcolic acid) without the use of organic solvents Biomaterials 17:1417-1422,(1996)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









