About Authors:
Abdulrhman A. Akasha
Department of Pharmaceutics, Faculty of Pharmacy,
Tripoli University, Libya-13645
akashaabdu@yahoo.co.uk
Abstract
Bioerodible microspheres may provide a vehicle for achieving good aerosolisation characteristics, whilst offering a slow release depot from the pulmonary deposited fraction. Polysebasic anhydride (PSA) was synthesised by a melt-polycondensation process using nitrogen gas under high vacuum. The products were characterised by 1H-NMR (2 & 20 KDa) and DSC (melting point: 68 & 83 °C), and with a yield (81 & 89 %) of prepolymer and polymer respectively. The polyanhydride was converted into microspheres using an oil in oil emulsification procedure. The polymer was dissolved in dichloromethane and using 1 to 5% Span 85 or oleic acid as stabilizer, dispersed into silicone oil. The dichloromethane was removed by evaporation, the microspheres recovered and washed with petroleum ether. Laser diffraction analysis of the microspheres indicated a mean microspheres size <5 mm. The presence of rough non-porous spheres was demonstrated by SEM. Initial in vitro experiments were undertaken to examine the degradation rate in 0.1 M phosphate buffer at 37 °C, pH 7.4. The process was followed for 24 hr, by which time 73% of the microsphere mass had eroded. However release of salbutamol base occurred more rapidly with 95% lost within 6-10 hr. The fact that 74% was released in the first 10 min suggest a high fraction of surface adsorbed drug. Microcarrier systems for slow release may provide a vehicle for achieving good aerosolisation characteristics, whilst offering a slow release depot from the pulmonary deposited fraction. The produced rough and non-porous microspheres containing salbutamol base were subjected for Deposition process using Andersen Mark II Cascade Impactor. The Deposition was assessed from both a Spinhaler and Rotahaler after 5s activation at a flow rate of 28.3L/min. This resulted in an average of 33.5% (Rotahaler and 17.5% (Spinhaler) of the dose found between stages 2 and the filter corresponding to microspheres in the size range 4.7 to 0.4 mm.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1382
1. Introduction
An aerosol may be defined as any system of solid particle or liquid droplets dispersed in air, having sufficiently small particle size to display considerable stability as a suspension. Bioerodible microspheres may provide a vehicle for achieving good aerosolisation characteristics, whilst offering a slow release depot from pulmonary deposited fraction. Polysebacic anhydride (PSA) was synthesized by a melt-polycondensation pocess [1]and used to prepare bioerodible microspheres by the solvent removal technique [2]to produce particles of a size small enough to be delivered to the lung (<5 mm). Biodegradable polymers constitute one of the most promising approaches to prolong the duration of action of drugs. They are favoured components of drug delivery systems because they do not possess some of the problems associated with many of the non-degradable systems. Various biodegradable polymers have been prepared for controlled of antihypertensive agents (Diltiazem) [3], controlled drug delivery of chemotherapy [4]and protein loaded polyanhydride micospheres for controlled delivery [5],[6]. The goal has been to develop implantable or injectable devices that provide controlled release of the drug for a defined period of time. Release of active drug from biodegradable systems can be occur by various mechanisms, including simple diffusion through the plymer membrane or matrix, polymer erosion, or a combination of diffusion and erosion and cleavage of covalent bond between polymer and drug.
The most commonly employed biodegradable polymers that have been used for microsphere fabrication include poly (lactic) and poly (glycolic) acid [7], polyacrylic acid [8], ethylcellulose [9], Ricinolic acid and lactic acid copolymer [10] and polyanhydride [11]. All are suitable for use as implantable or injectable release carriers.
Polyanhydride is a polymer that is composed of monomer units connected by anhydride bonds. This bond is labile to hydrolysis and break down into carboxylic acids groups. The structure of monomers plays a critical role in determining the properties of the polymers. Hence, intelligent selection of monomers is a crucial element in the development of biomedically useful polymer.
Polyanhydride may exist as homo or copolymer. The latter currently enjoys the most widespread interest in its medical applications. A common approach in designing biodegradable systems is to use two different kinds of monomers e.g. sebacic acid (SA) would completely erode within a few days, and 1,3-bis [p-carboxyphenoxy]-propane (CCP) is added to the polymer system to synthesise a copolyanhydride, which takes longer to degrade[12].
A preparation of biodegradible microspheres containing entrapped drug may provide good aerosolisation characteristics, whilst offering a slow release depot from pulmonary deposited fraction [13],[14]. Synthetic polysebacic anhydride (PSA) polymer has been chosen as fabricated biodegradible polymer from which microspheres will be synthesized by the solvent removal technique [2]. It will necessary to control the fabrication process to ensure that microspheres in an appropriate size range are produced (<5 mm in diameter). Biodegradable polymers constitute one of the most promising approaches to prolong the duration of action of drugs.
It is important feature in design of therapeutic aerosol that the aerosol particles containing the drug to be generated within the size range that will be deposited in the lower regions of the lung [15],[16]&[17]. The powder inhalation system depends entirely upon the patient’s inspiratory effect are generally easier to use than pressurised aerosols [18]. The first successful dry powder inhaler was the Spinhaler [19], which consists of tube containing a rotor into which gelatine capsule containing powdered drug is inserted by patient. Rotahaler is anther powder inhaler has been used to dispense the drug from gelatine capsule into the air stream. In the rotahaler the gelatine capsule is placed into the end of inhaler. As the end of the rotahaler is rotated, the capsule is broken into two halves. The drug contents are the available for aerosolization by inhaled air though the device.
[adsense:468x15:2204050025]
In-vitro deposition of microspheres containing salbutamol was studied using an Andersen Mark II Cascade Impactor. It consists of a series of up to eight metal stages with a collection disc positioned directly below each stage. Each stage represents an increasingly smaller aerodynamic diameter. The particles are introduced into the system via a moving air stream. The particles cascade through the increasingly smaller stages until they impacted as a function of their aerodynamic diameter. The amount of drug impacted on each stage was quantified as a relative percentage of total drug impacted on all the stages. The cascade impactor operates at flow rate of 28.3 L/min, which causes particles to fractionated according to their inertia into nine fractions. 10 mm and greater are retained in the pre-separator, the remaining being retained on eight stages with 50% cut-off diameter of 9, 5.8, 4.7, 3.3, 2.1, 1.1, 0.7, and 0.4 mm. Any aerosol particles small enough to avoid impaction within the device is collected on the terminal filter [20].
2. Materials and methods
2.1 Synthesis of Polysebacic anhydride
Polysebacic anhydride was synthesized by melt-polycondensation process as previously described by [1]. The prepolymer was prepared by refluxing the sebacicic acid, supplied by Fisons Laboratory Reagents, UK. with acetic anhydride obtained from Sigma Chemical Co, UK. for 20 minutes. The prepolymer then underwent melt-polycondensation at 180 °C and a high vacuum was applied. The polymerisation process was continued for 90 minutes. The products were characterised using 1HNMR (NMR spectroscopy (300 MHZ)) and DSC (Perkin-Elmer DSC7 differential scanning calorimeter).
2.2 Preparation of Polysebacic anhydride microspheres
The microspheres werte prepared by the solvent removal technique as described by [2]. The polymer (PSA) was dissolved in dichloromethane. The desired amount of drug (Salbutamol base, supplied by Glaxo Group Research, UK. was dissolved in dichloromethaneand surfactant (oleic acid) was added and 0.25 to 0.5ml of petroleum ether added. The mixture was added to silicone oil containing 1-5% span 85 as emulsifier and stirred at 20500 rpm, using an Ultra Turrax (Janke & Kunkel, IKA labrotechnik) for 10 minutes. The stirring was the continued using an over head stirrer (2000 r/m) for 4 to 5 h until the microspheres were produced. The microspheres were isolated by filtration, washed twice with petroleum ether and dried over night under vacuum. A Malvern 2600HSD laser diffraction particle sizer was employed for size analysis of microspheres. The surface morphology of microspheres was studied using scanning electron microscopy (SEM Philips Xl-20).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2.3 In-Vitro drug release and polymer degradation
Appropriate amounts of microspheres containing incorporated salbutamol base were placed in a series of tubes containing 0.1 M, pH 7.4 phosphate buffer. The samples were incubated and gently agitated on an end –over-end shaker at 22 rpm. Samples were taken periodically and centrifuged at 3500 rpm. The supernatant was analysed at 276 nm for salbutamol baseusing UV-1201 spectrophotometer, (Shimadzu).
Degradation rates were also measured by determing the microspheres’ weight loss during the same experiment. After the samples were centrifuged, the microspheres were freeze dried and re-weighed to calculate the weight loss.
2.4 In-vitro deposition of microspheres containing salbutamol base
Microsphere deposition was assessed by aerosolization using Andersen Mark II Cascade Impactor and a vacuum pump. The pump was calibrated and a flow rate of 28.3 L/min-1 used.
The weighed microspheres containing incorporated Salbutamol base (supplied by Glaxo Group Research, UK) were packed in a Spinhaler (Fisons, U.K) or Rotahaler (Glaxo, U.K). The Dry Powder Inhaler (DPI) was activated and aerolisation was undertaken for 5 seconds. The microspheres were collected from different stages for drug content analysis using glass ptri dishes, where the particles dissolved in 1 ml dichlormethane (Fisons, U.K), followed by addition of 10 ml phosphate buffer. The aqueous samples were analysed spectrophotometrically at 276 nm using UV-1201 spectrophotometer (Shimadzu).
3. Results and Discussion
The PSA was characterized by DSC and 1HNMR as shown in table 1. & figure1 and 2. And was used successfully to produce microspheres with a mean diameter of less than 5 mm. SEM micrographs showed a mean relatively smooth and non-porous spheres as shown in figure 3 and 4. This study was used the same methods of characterization of PSA as made by [21]and [22].
This study shows that PSA had melting point 82 ºC and molecular weight of 20.000 Da. This value considered to be a low molecular weight polymer. It is noteworthy, however, to point out that this investigation uses an analytical method derived from 1HNMR, which has been used to calculate the molecular weight of PSA. The method involves using an oil emulsion procedure has produced well-defined microspheres. The surface structure of PSA microspheres was studied using SEM. The electron microscopical observation reveal that the drug-containing micospheres in general appear reasonably smooth and non-porous and that they are spherical in shape figure 4. It has been reported that the microspheres prepared from low molecular weight polymer materials are generally non-porous rather than those prepared of high molecular weight would appear porous [23].
Table 1. Prepolymer and polymer characterizations
Peak integration: Chemical shift in ppm of methyl-terminal and representative peak of repeating unit.
CH3/CH2: The integration ratio in ppm of methyl-terminal to representative peak of repeating methylene units.
DP (degree of polymerization): Based on 1HNMR analysis.
|
|
DSC |
1HNMR |
M.Wt (KDa) |
|||
|
|
M:P (ºC) |
DH (J/g) |
Peak-integration |
ratio CH3/CH2 |
DP |
|
|
Pepolymer |
68-75 |
36.01 |
2.19-1.29 |
3:20.4 |
10.2 |
2.02 |
|
Polymer |
83 |
113.41 |
2.32-1.42 |
3:216 |
108 |
20 |
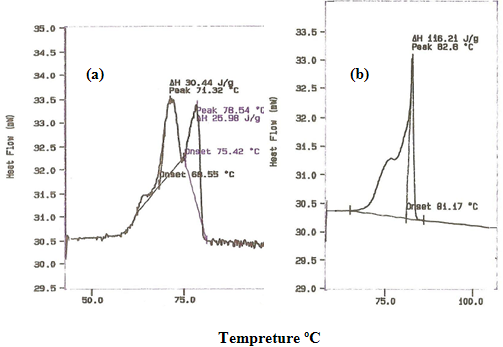
Figure 1. DSC traces of (a) Polysebacic anhydride prepolymer & (b) Polysebacic anhydride polymer.
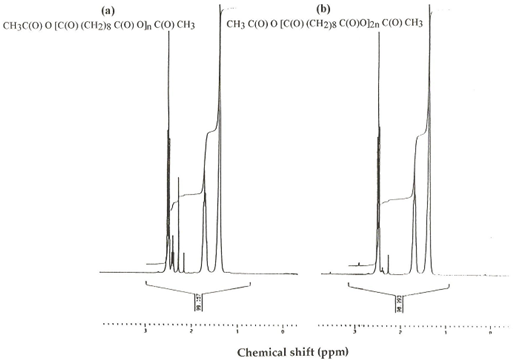
Figure 2. 1HNMR of (a) Polysebacic anhydride prepolymer & (b) Polysebacic anhydride polymer.
Figure 3. Size distribution analysis of Poysebacic anhydride (PSA) containing salbutamol base measured by Malvern laser diffraction.
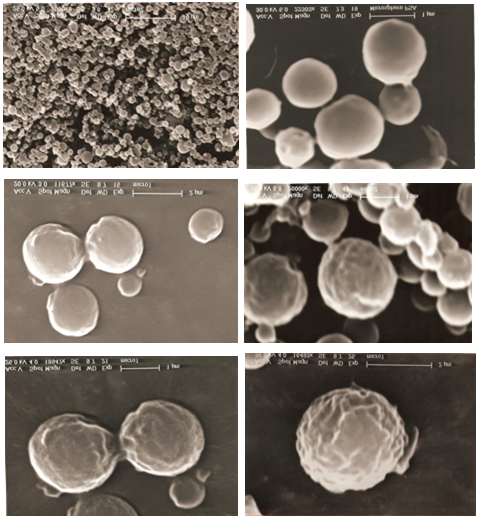
Figure 4. SEM micrographs of Poysebacic anhydride (PSA) containing salbutamol base prepared by solvent removal method, with different magnifications
The slow release of salbutamol may support the view that, the drug is distributed in both deeper inside the microspheres and superficially attached on the surface of micrspheres. Since 75% of salbutamol base was released within 10 minutes, this observation suggests a high percentage of drug was entrapped on the surface of PSA microspheres and that only a smaller proportion (25%) is encapsulated inside the PSA microspheres. This observation suggests that the high percentage release of salbutamol within such a short period time may be attributable to its hydrophilicity as shown in table 2. & figure 5. .
The degradation rate of PSA was followed for 24 hr by which time 73% of the microsphers’ mass had eroded. However, the release of salbutamol base occurred more rapidly than the erosion of PSA as shown in table 2. & figure 5. It seems that the slow degradation of salbutamol-loaded microspheres is due to the chemical rather than physical properties of salbutamol base since both druf and polymer are hydrophilic. The remaining PSA microspheres material were characterised as small brittle fragments of drug polymer complex.
Polysebacic anhydride microspheres were prepared previously by the solvent removal technique. The biodegradable microspheres were successfully produce small non-porous microcarriers with the size 5 mmand capable to contain drug for pulmonary drug delivery figure5. as investigated by SEM.
Table 2. In-vitro release of salbutamol base and degradation rate of polysebacic anhydride micospheres
|
Time (h) |
0.16 |
0.1 |
3.0 |
6.0 |
10.0 |
22.0 |
24.0 |
|
% Salbutamol release |
74.5 |
79.8 |
90.4 |
95.2 |
95.7 |
98.4 |
98.4 |
|
% Weight of microspheres remaining |
90.0 |
65.0 |
50.0 |
40.0 |
39.0 |
30.0 |
27.0 |
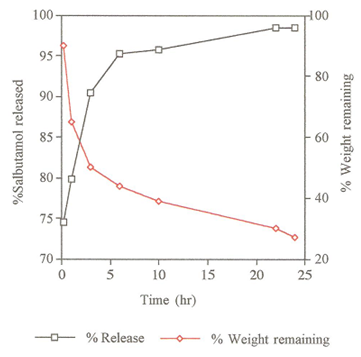
Figure 5. Weight loss and release rate of salbutamol base from PSA Microspheres.
In-vitro deposition of the biodegradable microspheres containing salbutamol base was assessed from both Spinhaler and Rotahaler following 5 seconds activation through an Andersen Mark II Cascade Impactor operating at a flow rate of 28.3 L/min-1. The results are summarised in table 3 and 4 and in the form bar-graphs in figure 6 and 7. The microspheres were separated into nine fractions plus the filter retension of particles <0.4 mm. An approximately 60% and 80% of emitted dose of microspheres were found with an effective cut-off diameter > 5 mm for Rotahhaler and Spinhaler respectively, whereas standard 75% of commercially available DPI. 33.5% and 17.5 of total dose was deposited between stage 2 and filter for Rotahhaler and spinhaler respectively, compared with approximately 25% of deposited standard salbutamol [16].
It has been found that the Rotahaler, 27% of the particles are in the preseparator with decreasing amounts deposited on progression down the impactor. The filter which is at the bottom of the impactor contains 2.7% of the microparticles. However, the use of a spinhaler produce a different pattern of microparticles distribution. In the case, 44.4% of microparticles were found in the preseparator and 0.8% in the filter.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 3. In-vitro deposition of Polysebacic anhydride (PSA), microspheres containing salbutamol base in Cascade impactor after 5 seconds activation using Rotahaler.
|
Fraction |
Average (%) ±SD (N=3) |
|
Preseparator |
27.0 ±5.8 |
|
0 |
21.0 ±2.0 |
|
1 |
11.8 ±1.1 |
|
2 |
7.30 ±1.7 |
|
3 |
6.50 ±1.9 |
|
4 |
5.60 ±0.8 |
|
5 |
5.70 ±1.0 |
|
6 |
6.70 ±2.3 |
|
7 |
4.60 ±0.6 |
|
F |
2.70 ±0.3 |
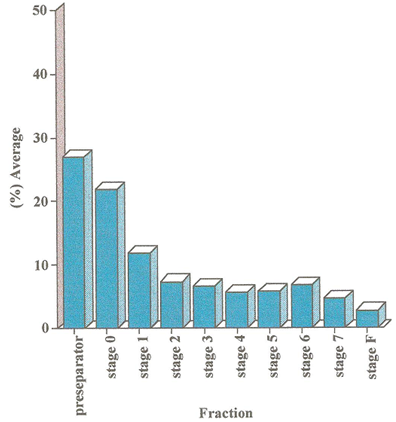
Figure 6. In-vitro deposition of Polysebacic anhydride (PSA), microspheres containing salbutamol base in Cascade impactor after 5 seconds activation using Rotahaler.
Table 4. In-vitro deposition of Polysebacic anhydride (PSA), microspheres containing salbutamol base in Cascade impactor after 5 seconds activation using Spinhaler.
|
Fraction |
Average (%) ±SD (N=3) |
|
Preseparator |
44.4 ±5.8 |
|
0 |
28.0 ±4.3 |
|
1 |
9.90 ±1.7 |
|
2 |
5.20 ±0.7 |
|
3 |
3.70 ±0.1 |
|
4 |
2.10 ±0.3 |
|
5 |
1.76 ±0.1 |
|
6 |
1.40 ±0.6 |
|
7 |
2.22 ±0.6 |
|
F |
0.80 ±0.2 |
Figure 7.In-vitro deposition of Polysebacic anhydride (PSA), microspheres containing salbutamol base in Cascade impactor after 5 seconds activation using Spinhaler.
It is not surprising to find that there were differences between the particle size of microspheres measured by laser diffraction and the particle size measured using the Anderson Cascade Impactor. The large particle size determined using the Anderson Cascade Impactor may be explained by incomplete deaggregation of microspheres aggregates, which form during capsule loading, on arosolization using Rotahaler or Spinhaler.
According to the investigation [15], the microparticles having diameter larger than 10 mm are retained in the preseparator of the Anderson Cascade Impactor and those of smaller sizes are distributed in eight stages plus the filter retention of particles < 0.4%. A gradual decrease in the number of particles on a descending stage of impaction was observed in this study. The later investigation demonstrated that particles with diameters less than 4.7 mm were impacted on stage 2 and the rest of particles are deposited on different stages with each representing an increasingly smaller aerodynamic diameter. Furthermore, this study reveals that of the total number of particles, 33% in case of Rotahaler and 17.5% in case of Spinhaler were deposited on stages between stage 2 and the filter.
4. Conclusions
The study provides evidence to support the view that microspheres prepared from low molecular weight polymer such as PSA are non-porous.
For pulmonary drug delivery system, it is important to use small microspheres as the drug carriers. The microspheres produced by these methods are of reasonably small size, ranging from 1mm to 5 mm. The process involves the use of a high stirring speed of 20500 rpm.
The addition of surfactant to the organic solvent containing drug followed by the mixture of polymer and organic solvent is also paramount importance as this is found to increase the solubility and thus to enhance drug infiltration into the polymer matrix.
This results in a better entrapment of drug inside the microspheres and slower release.
Acknowledgements
I would like to acknowledge and thankdepartment of pharmaceutics, Welsh School of Pharmacy, University of Wales, Cardiff, United kingdom, for providing facilities for this study and Libyan high education for generous funding.
References
1. Domb AJ, Carlos FC, Langer R. Polyanhydrides Based on Aliphatic-Aromatic Diacids. Macromol 1989; 22:3200-3204.
2. Mathiowitz E, Carmela Amato, Ph Dor, Langer R. Polyanhydrides microspheres: 3. Morphology and characterization of systems made by solvent removal. Polymer 1990; 31:547-555.
3. Farhana SA, Shantakumar SM, Narasu L. Sustained release of diltiazem hydrochloride from chitosan microcapsules. Curr Drug Deliv 2009; 6:238-248.
4. Sampath P, Berm H. Implantable slow release chemotherapeutic polymers for treatment of malignant tumors. Cancer Control 1998; 5:130137.
5. Tabata Y, Gutta S Langer R. Controlled delivery systems for proteins using polyanhydride microspheres. Pharma Res 1993; 10: 487-496.
6. Sun L, Zhou S, Wang W, Su Q, Li X. Preparation and characterization pf protein-loaded polyanhydride microspheres. J Mat Sci 2009; 20: 2035-2042.
7. Valizadeh H, Mohammadi G, Ehyaei R, Milani M, Azharzdeh M, Zakeri-Milani P, Lotfipour F. Antibacterial activity of clarithromycin loaded PLGA nanoparticles. Pharmazie 2012; 67:63-68.
8. Greimel A, Werle M, Bernkop-Schnürch A. Oral peptide delivery: in-vitro evaluation of thiolated alginate/poly (acrylic acid) microparticles. J Pharm Pharmacol 2007; 59:1191-1198.
9. Shailesh T, Vioul P, Girishbhai J, Manish C. Preparation and in vitro Evaluation of Ethylcellulose Coated Egg Albumin Microspheres of Diltiazem Hydrochloride. J Young Pharm 2010; 2:27-34.
10. Slivaniak R, Ezra A, Domb AJ. Hydrolytic degradation and drug release of ricinoleic acid-lactic acid copolyesters. Pharm Res 2006; 13:1306-1312.
11. Slager J, Tyler B, Shikaov A, Domb AJ, Shogen K. Local controlled delivery of anti-neoplastic RNAse to the brain. Pharm Res 2009; 26: 1838.1846.
12. Shieh L, Tamada J, Tabata Y, Domb AJ, Langer R. Drug release from a new family of biodegradable Polyanhydride. J Controll Rel 1994; 29:73-82.
13. Devrim B, Bozk?r A, Canefe K. In vitro studies on aerodynamic properties of dry powder inhaler formulations. Drug Dev Ind Pharm 2011;37:1376-1386.
14. Yang Y, Bajaj N, Xu P, Ohn K, Tsifansky MD, Yeo Y. Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 2009; 30:1947-53.
15. Moazeni E, Gilani K, Sotoudegan F, Pardakhty A, Najafabadi AR, Ghalandari R, Fazeli MR, Jamalifar H. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul 2010;27:618-27.
16. Nolan LM, Li J, Tajber L, Corrigan OI, Healy AM. (2011), Particle engineering of materials for oral inhalation by dry powder inhalers. II-Sodium cromoglicate. Int J Pharm. 2011 Feb 28;405(1-2):36-46.
17. Abdelrahim ME. Aerodynamic characteristics of nebulized terbutaline sulphate using the Andersen Cascade Impactor compared to the Next Generation Impactor. Pharm Dev Technol 2011; 16:137-45.
18. Stahlhofen W, Gebhart J, Hyder J. Experimental determination of the regional deposition of aerosol particles in the human respiratory tract.Am Ind Hyg Assoc J 1980; 41: 385-388
19. Bell JH, Hartley PS, Cox JSG. Dry powder aerosols. I. A new powder inhalation device. J Pharm Sci 1971; 60:1559-1563.
20. Kawashima Y, Yamamoto H, Takeuchi H, Fujioka S, Hino T. Pulmonary delivery of insulin with nebulized DL-lactide/glycolide copolymer (PLGA) nanospheres to prolong hypoglycemic effect. J Controll Rel 19991; 62:279-87.
21. Dang W, Daviau T, Berm H. Morphological characterization of polyanhydride biodegradable implant gliadel during in vitro and in vivo erosion using scanning electron microscopy Pharm Res. 1996; 13: 683-691.
22. Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Sythesis and characterization of novel Polyanhydride with tailored erosion mechanism. J Bio Mater Res A 2006; 76:1202-1210.
23. Bindschaedier C, Leang K, Mathiowitz E, Saltzman WM, ,Langer R. Polyanhydride microsphere formulation by solvent extraction. J Pharm Sci. 1988; 77:696-698.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









