ABOUT AUTHORS:
Girish Kumar Tripatha*, Satyawan Singha
Bio-organic chemistry Laboratory,
Saroj Institute of Technology & Management Lucknow, India
*orgpharm@gmail.com
ABSTRACT:
Undulating developments in the field of medical science have revolutionized the research in the field of advanced drug delivery. Polymers show usually an improved pharmacokinetics compared to small molecule drugs with longer circulation time and the potential for tissue targeting. There is a growing interest in developing stimuli-sensitive polymers that can recognize and respond to biologically-relevant stimuli so they can be used as carriers for tissue and cell-specific drug delivery. Stimuli-sensitive polymers play an important role in the development of advanced delivery vehicles that can effectively deliver a wide range of therapeutic molecules including nucleic acids, peptides, proteins etc. Stimuli responsive polymers show a sharp change in properties upon a small or modest change in environmental condition. The most important stimuli are pH, temperature, ionic strength, light and redox potential. This review article will focus on different stimuli sensitive polymer and pH stimuli sensitive drug delivery used as carrier for the targeting of drugs.
REFERENCE ID: PHARMATUTOR-ART-1889
1. INTRODUCTION
Polymers are macromolecules having very large chains, contain a variety of functional groups, can be blended with other low and high molecular-weight materials, and can be tailored for any applications. Polymers have been used as a main tool to control the drug release rate from the formulations. Extensive applications of polymers in drug delivery have been realized because polymers offer unique properties which so far have not been attained by any other materials. Synthetic polymers are being used as drug. Delivery systems as a polymeric drug itself or in combination with small molecule drugs or with biomacro molecules such as proteins and poly stimuli-responsive polymers mimic biological systems in crude way where an external stimulus (e.g. change in pH , temperature etc.) results in a change in properties. This can be a change in conformation, solubility, alteration of the hydrophilic/hydrophobic balance or release of a drug. This behavior can be utilized for the preparation of so called smart drug delivery systems, which mimic biological response behaviour to a certain extent.
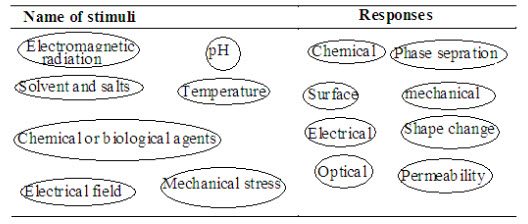
The polymer is not a drug itself; it often provides a passive function as a drug carrier, reducing immunogenicity, toxicity or degradation, whilst improving circulation time and potentially a passive targeting function. Stimuli responsive polymers have been used in a large variety of applications, even though there are systems, which show a linear response to an external stimulus, it is more interesting to study those polymers with a non-linear behavior, because bio-logical systems also accomplish specific setting of environmental conditions in different parts of the body (Table 1). This means that a polymer exhibits a large change in properties (response) as a result of a small change in environmental conditions (stimulas), which is in addition often reversible.
Figure 1. Potential stimuli and responses of stimuli sensitive polymer
Human body presents variations on pH along the gastrointestinal tract, and also in some specific areas like certain tissues (tumoral areas etc) or sub-cellular compartments. pH changes within the body and it can therefore be used to direct the response to a certain tissue or cellular compartments [1]. The changes along the gastrointestinal tract from acidic in the stomach (pH = 2) to basic in the intestine (pH = 5-8) has to be considered for oral delivery of any kind of drug [2]. Certain cancers as well inflamed or wound tissue exhibit a pH different from 7.4 as it is in circulation. For example chronic 7.4 and 5.4 and cancer tissue is also reported to be acidic extracellularly [2, 3].
Table: pH in various tissues and cellular compartments [2]
|
S.N. |
Tissue/cellular compartment |
pH |
|
1. 2. 3. 4. 5. 6. 7. 8. 10. |
Blood Stomach Duodenum Colon Early endosome Late endosome Lysosome Golgi Tumour,extracellular |
7.35-7.45 1.0-3.0 4.8-8.2 7.0-7.5 6.0-6.5 5.0-6.0 4.5-5.0 6.4 7.2-6.5 |
The aim of this article is to review advances in stimuli-responsive polymers in drug delivery. It will substantiate how polymers can be used in a smart fashion potentially leading to multiple responses at the desired point of action.
2. Stimuli sensitive polymers
Stimuli sensitive polymers are non-thrombogenic, biocompatible, strong, flexible, tough, increase patient compliance, maintain stability of the drug and maintain drug level in therapeutic window. Stimuli sensitive polymers are composed of polymers that respond in a dramatic way to very slight changes in the environment or they may be defined as plastics which change or react in a certain way according to the environment. They are also known as ‘smart polymers or intelligent polymers’ or ‘environmental-sensitive polymers .The characteristic feature that actually makes these polymers ‘smart’ is their ability to respond to very slight changes in the surrounding environment. The uniqueness of these materials lies not only in the fast microscopic changes occurring in their structure but also these transitions being reversible. The responses are manifested as changes in one or more of the shape, surface characteristics, and solubility etc. The environmental trigger behind these transitions can be either change in temperature or pH shift, increase in ionic strength, presence of certain metabolic chemicals, addition of an oppositely charged polymer and poly cation poly anion complex formation, changes in electric, magnetic field, and light or radiation forces. Smart polymers are becoming increasingly more common, as scientists learn about the chemistry and triggers that induce conformational changes in polymer structures and create ways to take advantage of them, and eventually control them. New polymeric materials are being chemically formulated that sense specific environmental changes in biological systems, and adjust in a predictable manner, making them useful tools for drug delivery or other metabolic control mechanisms.
2.1.Classification of stimuli sensitive polymers
The stimuli sensitive polymers are broadly classify under following categories:
a. Temperature sensitive smart polymers
b. Polymers with dual stimuli-responsiveness
c. Phase sensitive smart polymers
d. Light sensitive smart polymers
e. pH sensitive smart polymers
a. Temperature sensitive smart polymers
These are those polymeric systems which are sensitive to temperature changes and polymers show gel-to-gel transition as a function of environmental temperature which can be utilized to deliver therapeutic agents in vivo. These types of systems exhibit a critical solution temperature at which the phase of polymer and solution is changed in accordance with their composition. Many polymers show abrupt changes in their solubility as a function of environmental temperature. This property was employed to develop aqueous solutions of these polymers which undergo sol-gel transition in response to temperature changes. Examples of polymers that show temperature sensitive character are poly (N-isopropylacrylamide), poly (ethylene oxide)- poly (propylene oxide)- poly (ethylene oxide) triblock copolymers, poly (ethylene glycol)- poly (lactic acid)- poly (ethylene glycol) triblocks (PEG-PLA-PEG). The most widely used temperature sensitive polymers include poly (N-alkyl substituted acrylamides) and poly (N-isopropyl acrylamide) with transition temperature of 32 0C and poly (Nvinylalkylamides) like poly (N-vinyliso-butyramide) with transition temperature of 39 0C [4]. Mechanism of action of temperature-sensitive smart polymeric solubility usually originates from the existence of a lower critical solution temperature beyond which the polymer becomes insoluble in water. Such behavior is typical for the polymers that form hydrogen bonds with water and has wide range of biological applications such as cell patterning, smart drug release etc.
b. Polymers with dual stimuli- responsiveness
These are the polymeric structures sensitive to both temperature and pH, they are obtained by the simple combination of ionisable and hydrophobic (inverse thermo-sensitive) functional groups. Mechanism of action of polymers with dual stimuli-responsiveness is mainly achieved by the copolymerization of monomers bearing these functional groups, combining temperature sensitive polymers with polyelectolytes or by the development of new monomers that respond simultaneously to both stimuli [5] .Examples of dual stimuli-responsive polymers with their application:
(i). The major application of polymers with dual stimuli-responsiveness is the formation of several smart core-shell microgels based on poly (N-isopropylacrylamide), polymethacrylic acid and chitosan or poly (ethyleneimine) in the absence of surfactants. These materials were obtained by graft copolymerization and presented a well defined core-shell structure consisting of temperature-sensitive cores with pH-sensitive shells.
(ii). Second major application of these smart polymers is the formation of elastin-like polymers by genetic engineering. These materials were developed by fermentation, which showed clear environmental advantages. The elastin-like polymers presented a modulated pH- and light sensitive molecules.
c. Phase sensitive smart polymers
Phase sensitive smart polymers can be used to develop biocompatible formulations for controlled delivery of proteins in a conformationally stable and biologically active form. These smart polymeric systems have many advantages over other systems such as ease of manufacture, less stressful manufacturing conditions for sensitive drug molecules, and high loading capacity [6].
This approach employs a water insoluble biodegradable polymer, such as poly (D, L-lactide), poly (D, L-lactide-co-glycolide) and poly (D, L-lactideco- e-caprolactone), dissolved in pharmaceutically acceptable solvent to which a drug is added forming a solution or suspension. After injection of the formulation in the body, the water-miscible organic solvent dissipates and water penetrates into the organic phase. This causes phase separation and precipitation of the polymer forming a depot at the site of injection. Organic solvents used include hydrophobic solvents, such as triacetin, ethyl acetate, and benzyl benzoate, and hydrophilic solvents, such as N-methyl-2-pyrrolidone, tetraglycol, and glycofurol [7]. Examples of phase sensitive smart polymers with their application:
(i). Lysozyme release
Major application of phase sensitive smart polymer is the lysozyme release. This type of phase sensitive systems were prepared by adding lysozyme to poly (D, L-lactic acid) (PLA)-triacetin solutions [8].
(ii).Controlled release of proteins
Phase sensitive smart polymeric formulations have wide application for the controlled release of several proteins. One of the problems is the burst releasing during the few hours following injection into the body. This could be due to the lag time between the injection of the delivery system and the formation of the gel depot. These polymeric systems are also known to display a high initial release of the drug followed by a more sustained release profile. The initial high burst release follows the Higuchi square root of time relationship. The solvents used in phase sensitive smart polymeric systems should be nontoxic and biocompatible to avoid severe tissue irritation or necrosis at the site of administration.
The application has been explored by using of emulsifying agents in phase sensitive formulations. When the emulsifying agent is mixed with the viscous gel, the emulsifying agent forms a separate phase of microscopic-size dispersed droplets. The continuous phase is formed of the polymer and the solvent. The bioactive agent can be dissolved or dispersed in either the continuous phase or the droplet phase. This formulation has an advantage of increased stability of the drug. Preferred emulsifying agents include alcohols, propylene glycol, glycerol, and water.
d. Light sensitive smart polymers
Light can be applied as an external stimulus to switch drug release on and off at a specific site, offering a potential for release on demand in the targeted places of the human body that is otherwise difficult to achieve using other stimuli. A visible light sensitive smart polymer that forms aqueous two phase systems are potentially used in industrial bioseparation techniques. Many of the problems of two phase systems like; they cannot be recycled, result in increasingly expensive bioproducts, purification processes and environmental pollution have been overcome by the use of light sensitive smart polymers.These systems are biocompatible, biodegradable, polymerizable, and at least partially water soluble macromers. The macromers include at least one water soluble region, at least one region which is biodegradable and at least two free radical polymerizable regions. Macromers are polymerized by free radical initiators under ultraviolet light, visible light excitation, or thermal energy. The core water soluble region can consist of PEG, poly (vinyl alcohol), polysaccharides such as hyaluronic acid, and proteins such as albumin. The biodegradable regions may be polymers made up from polylactic acid, polyglycolic acid, poly(anhydrides), poly (amino acids) and polylactones. Preferred polymerizable regions include acrylates, diacrylates, methacrylates or other biologically accepted polymerizable groups [9].
e. pH sensitive smart polymers
pH sensitive smart polymers are polyelectrolytes that bear in their structure weak acidic or basic groups that either accept or release protons in response to changes in environmental pH. In case of pH sensitive polymers, swelling of polymer increases as the external pH increases in the case of weakly acidic (anionic) groups also known as polyacids, but decreases if polymer contains weakly basic (cationic) groups termed as polybases.Most of the anionic pH sensitive smart polymers are based on polyacrylic acid, carbopol or its derivatives, polymethacrylic acid, poly(ethylene imine), poly (L-lysine), and poly (N, N-dimethylaminoethyl methacrylamide). The another kind of poly acidic polymer is the polysulfonamides i.e. derivatives of p-amino benzene sulfonamide [10]. Examples of cationic polyelectrolytes are poly (N, N-dialkyl aminoethyl methacrylates), poly (lysine), poly (ethylenimine) and chitosan [11].
Oral pH sensitive drug delivery systems are gaining importance as these systems deliver the drug at specific part of the gastrointestine time as per the pH of gastrointestine, resulting in improved patient therapeutic efficacy and compliance [12]. The pH range of fluids in various segments of the gastrointestine tract (GIT) may provide environmental stimuli for the response drug release. The pH range of the gastric fluids varies in various segments of the gastrointestine tract, which may provide environmental stimuli to design of delivery system against Helicobacter pylori infection (Figure 2).
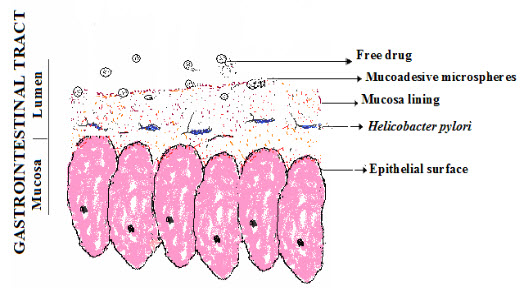
Figure 2. Schematic showing mode of interaction of drug loaded microspheres on targeted site of H. pylori infection.
Mechanism of action of pH sensitive smart polymers polyelectrolyte is a macromolecule that dissociates to give polymeric ions after dissolving in water or another ionizing solvent. All the pH sensitive smart polymers contain pedant acidic or basic groups on polyelectrolytes undergo ionization just like acidic or basic groups of monoacids or mono bases [13,14]. The ionizable groups act as hydrophilic part or hydrophobic part of the polymer. An acidic group of the polymer such as carboxylic acid is ionized at pH above pKa and deionized at pH below pKa. But a basic group such as amine is deionized at pH below pKb and ionized at pH above pKa. There are few pH sensitive polymers with their applications are given in following succeeding paragraph:
(i). Glucose sensors
Microparticles prepared by polymer poly(methacrylic acid-g-ethylene glycol) loaded with insulin was exhibited unique pH responsive characteristics in which interpolymer complexes were formed in acidic media and dissociated in neutral or basic environments.The insulin release from the gel was significantly retarded in acidic medium while rapid release occurred under neutral/basic conditions [15].
(ii). Modified drug delivery system
pH sensitive smart polymers consist of the polymers for which the transition between soluble and insoluble state is created by decreasing net charge of the polymer molecule. The net charge can be decreased by changing pH to neutralize the charges on the macromolecule and hence to reduce the hydrophilicity of the macromolecule.The co-polymerization of some specific monomers results in the synthesis of a pH sensitive smart polymer with reversible transition in the physiological range of pH 7.0-7.5, thus making them more suitable for biological systems. In another case, when pH sensitive smart polymeric chains are cross linked forming smart polymers, their behaviour is not only influenced by the nature of the ionizable groups, the polymer composition and the hydrophobicity of the polymer backbone, but also by the crosslinking density. This affects the solute permeability in terms of bioactive compound release in several applications [16, 17].
(iii). Sustained drug release
A different type of pH sensitive smart polymeric systems relates to sustained release formulations using alginate gel beads or particles. This approach involves the formation of sustained release gels by the co-precipitation of alginate gel beads with a biologically active agent. The main advantage of this approach is that it provides high loading of the drug.
Hence stimuli-sensitive polymers play an important role in the development of advanced delivery vehicles that can effectively deliver a wide range of therapeutic molecules including nucleic acids, peptides, and proteins. There is a growing interest in developing stimuli-sensitive polymers that can recognize and respond to biologically-relevant stimuli so they can be used as carriers for tissue and cell-specific drug delivery. The rapid advancement in the area of polymer synthesis and the increasing popularity of controlled polymerization techniques will further accelerate the development of new sophisticated carriers with well defined compositions that can exploit physiological signals to trigger the release of their therapeutic cargo in a controlled fashion. Further, it is expected that the next generation of stimuli-sensitive carriers will have a hybrid composition that combines polymeric structures with natural biological moite to further take advantage of natural transport mechanisms to reach their target tissue/cell as well as natural elimination pathways after the delivery of their cargo.
3. pH sensitive drug delivery system
3.1. pH-sensitive hydrogel
pH-sensitive hydrogels have been most frequently used to develop controlled release formulations for oral administration. The pH in the stomach is quite different from the neutral pH in the intestine and such a difference is large enough to elicit pH sensitive behaviour of polyelectrolyte hydrogels.The polymers with a large number of ionizable groups are known as polyelectrolytes. Fig.1.2 shows anionic and cationic polyelectrolytes and their pH-sensitive ionization. Poly (acrylic acid) becomes ionized at high pH, while poly (N, N-diethylamino ethyl methaacrylate) becomes ionized at low pH.
As shown in Fig. 3, cationic polyelectrolytes, such as poly (N, N -diethylaminoethyl methaacrylate), dissolve more or swell more if cross linked, at low pH due to ionization. On the other hand, polyanions, such as poly (acrylic acid), dissolve more at high pH.
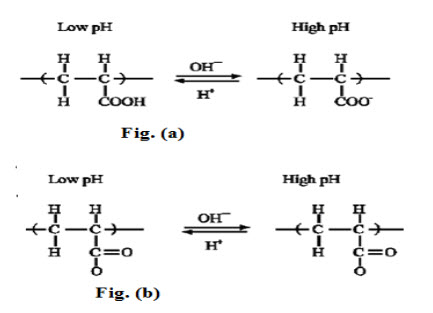
Fig.3. pH-sensitive ionization of polyelectrolyte (a) Poly(acrylic acid and (b) Poly (N, N-diethylaminoethyl methacrylate)
This property has been used to prevent release of foul-tasting drugs into the neutral pH environment of the mouth. pH-sensitive hydrogels have also been used in making biosensors and permeation switches [18]. The pH-sensitive hydrogels for these applications are usually loaded with enzymes that change the pH of the local microenvironment inside the hydrogels. One of the common enzymes used in pH-sensitive hydrogels is glucose oxidase which transforms glucose to gluconic acid. The formation of gluconic acid lowers the local pH, thus affecting the swelling of pH-sensitive hydrogels.
3.2. Enteric-coated systems
The enteric coating has been intended use included taste and odor masking, drug stabilization, protection against local irritation and release directed to defined segments in the gastrointestine tract. A major aim of enteric coating is protection of drugs that are sensitive or unstable at acidic pH (Fig.4). This is particularly important for drugs such as enzymes and proteins, because these macromolecules are rapidly hydrolyzed and inactivated in acidic medium. Antibiotics, especially macrolide antibiotics like erythromycin, are also rapidly degraded by gastric juices. Others, such as acidic drugs like NSAID’s (e.g., diclofenac, valproic acid, or acetylsalicylic acid) need to be enteric coated to prevent local irritation of the stomach mucosa.
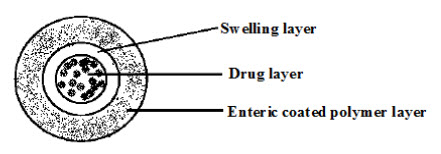
Fig.4.Schematic representation of enteric coated system
In general, film-coated dosage forms can be divided into multiple-unit and single unit dosage forms. Single units comprise tablets and film-coated capsules or other forms, usually monolithic structures. Multiple-unit dosage forms can be packages containing granules, capsules containing pellets, or compressed film-coated particles. Recently, it has also been reported that aqueous dispersions or suspensions can be produced, in which the drug is present in enteric-coated form. The enteric-coated time clock system consists of a tablet core coated with a mixture of hydrophobic material and surfactant, which is applied as an aqueous dispersion [19]. The drug release from the core of the time clock system occurs after a pre-determined lag time.
3.3. pH-sensitive gels
Many polyanionic materials, such as poly (acrylic acid), are pH sensitive and the degree of swelling of such polymers can be modulated by changing the pH. The use of these systems in conjunction with temperature-sensitive lipids offers potential to target drugs to areas of inflammation or to achieve site-specific, pulsatile drug delivery [20]. An environmentally responsive, hydrogel microsphere coated with a lipid bilayer has recently been shown to act as a secretory granule mimic (Figure 5). Methylene-bis-acrylamide/methacrylic acid anionic microgels were prepared by precipitation polymerization and loaded with doxorubicin and condensed by incubating in buffer at pH 5. The condensed particles were then coated with a lipid bilayer. Disruption of the lipid bilayer by electroporation was shown to cause the microgel particles to swell and release their drug.
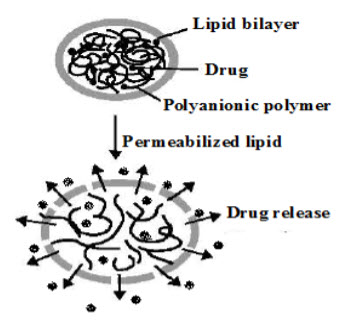
Figure 5. A schematic diagram showing the release of drug from a biomimetic secretory granule on disruption of the external lipid bilayer
3.4. pH-sensitive liposomes
The concept of pH-sensitive liposomes emerged from the observation that certain enveloped viruses infect cells following acidification of the endosomal lumen to infect cells and from the knowledge that some pathological tissues (tumors inflamed and infected tissue) have a more acidic environment compared to normal tissues (Figure 6). Although, pH-sensitive liposomes are stable at physiological pH, they destabilize under acidic conditions, leading to the release of their aqueous contents [21]. The liposomes are internalized by endocytosis after binding to cell surface receptors.
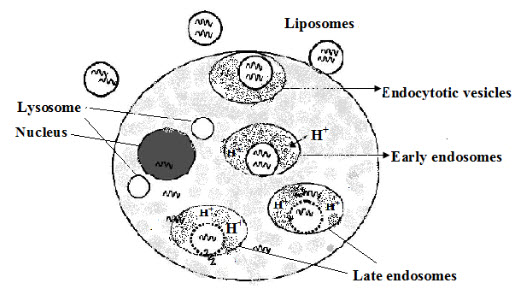
Figure 6.Intracellular delivery of oligonucleotides pH -sensitive liposomes
The potential of pH sensitive liposome lies in their ability to undergo destabilization at this stage, thus preventing their degradation at the lysosomal level [22].
3.5. pH sensitive microspheres
The pH of the human gastrointestinal tract was shown to increase progressively from the stomach, small intestine to the colon. It has been reported that the average gastric emptying time of multiple units was in the range of 1-3 hr in a fasted state and 2-4 hr in a fed state [23]. The small intestinal transit is surprisingly constant at 3-4 hr and appears to be insensitive of the type of dosage form and whether the subject is in the fasted or fed state [24]. It was reported that the changes in the pH of the gastrointestinal tract had a certain gradient and the transit time of materials through the gut was comparatively long. These findings provided the foundation for designing the pH-sensitive drug delivery system. Since the drug release persists throughout the whole gastrointestinal tract, this results in sustained transport of the drug and a prolonging of its pharmacological action in vivo.
3.6.pH sensitive nanoparticles
It was reported that particles in the size range 40-120 nm were translocated both trans-cellularly and para-cellularly [25].In addition to the potential for enhancing drug bioavailability via particle uptake mechanisms, particulate oral delivery systems can protect labile macromolecules from stomach acid and from the first-pass metabolism in the gastrointestinal tract. Likewise, particulate formulations also can increase transit times than larger dosage forms and can increase the local concentration gradient across absorptive cells. Thereby enhancing local and systemic delivery or both free and bound drugs across the gut [26].
pH sensitive polymer-based delivery systems have progressed to the clinical and in some cases to the commercial production. These delivery systems encounter many challenges associated with their development that are related to the drug stability, insensitive to the changing metabolic state, drug release kinetics and the conditions under which the system is delivered to the body. The under taken work focuse to develop pH sensitive, few antibacterial loaded mucoadhsive microparticulate system that would achieve continuous release of the drug in the gastric region and thus be useful for complete termination of the microbial infection from the gastric sites.
CONCLUSION
Stimuli-responsive polymers offer great advantages in drug delivery. The rapid advancement in the area of polymer synthesis and the increasing popularity of controlled polymerization techniques will further accelerate the development of new sophisticated carriers with well defined compositions that can exploit physiological signals to trigger the release of their therapeutic cargo in a controlled fashion. Further, it is expected that the next generation of stimuli-sensitive carriers will have a hybrid composition that combines polymeric structures with natural biological motifs to further exploit natural transport mechanisms to reach their target tissue/cell as well as natural elimination pathways after the delivery of their cargo.
Acknowledgement
Authors are thankful to Dr. P.R. Mishra , Scientist , Division of Pharmaceutics , Central Drug Research Institute Lucknow, India for his valuable support for this work.
REFERENCES
[1]. AT Florence; D Attwood, Physicochemical Principles of Pharmacy, 3rd Edition, Macmillan Press, London, 1998; 379- 380.
[2]. D.B. Beten and A.J. Moes. Controlled –release coevaprates of dipyridamole prepared with acrylic polymers.Int. J. Pharm., 1994, 103:243-251.
[3]. P Vaupel; F. Malinowski ; P. Okunieff, Cancer Res., 2006, 6699-6707.
[4]. YL Cao; C Ibarra, C; Vacanti, Tissue engineering: methods and protocols. , 1st Edition Humana Press, Totowa, 2006,75- 84.
[5]. L Lou ; M Kato; T Tsuruta ; K Kataoka; Y Nagasaki, Macromolecules J., 2000, 33: 4992-4994.
[6]. J Huang ; XY Wu, Polymer Sci. J., 1993, 37, 2667-2676.
[7]. T Higuchi, Pharm. Sci. J., 1963, 52, 1145-1149.
[8]. S Singh; J Singh, Int. J. Pharm, 2004 , 271, 189-196.
[9]. LH Gan ; YY Gan; DG Roshan Dean, Macromolecules J., 2000 , 33 ,7893-7897.
[10]. JF Hester; SC Olugebefola; AM Mayes, Membrane Sci. J., 2002, 208, 375-388.
[11]. SY Park;YH Bae, Macromoeculel Rapid Communication, 1999, 20, 269-273.
[12]. GK Tripathi; S Singh; G Nath; RK Dubey, Curr. Drug Deliv., 2011, 8, 667-677.
[13]. M Samer ; D Streich; W Meier, Advan. in Material, 2001, 13, 1649-1651.
[14]. Y Nagasaki; L Luol; T Tsuruta; K Kataoka , Macromol.Rapid Communication, 2000, 22, 1124-1127.
[15]. K Podual; H Doyle; N A Peppas, N. A.,Prepration and dynamic response of cationic co polymer hydrogel conaning glucose oxidase Cationic Polymer J., 2000, 41, 3975-3983.
[16]. E Kokufuta; YQ Zhang ;T Tanaka; A Mamada, Macromol. J., 1993, 26, 1053- 1059.
[17]. PS Stayton ; T Shimoboji ; C Long , Nature J., 1995 , 378: 472-475.
[18]. AS Hoffman, Intelligent Polymers, Controlled Drug Delivery: Challenge and Strategies. American Chemical Society, Washington, DC. 1997, 485-497.
[19]. RSS Wilding; F Davis; P Pozzi; A Furlani, Enteric coated timed release for colonic targeting. Int. J. Pharm., 1994, 111, 99-102.
[20]. S Hongkee; YH Chien ; H Park ; SJ Hwang ; K Park;AW Lloyd , Drug delivery and targeting in new generation technologies. Taylor and Francis, New York, 2001, 385-386.
[21]. H Ellens; J Bentz; FC Szoka, Biochem., 1984, 23, 1532-1538.
[22]. N Duzgunes; RM Straubinger; PA Baldwin; DS Friend; D Papahadjopoulos, Biochem., 1985, 24, 3091-3098.
[23]. SS Davis; JG Hardy; JW Fara, Gut, 1986, 27, 886-892.
[24]. PJ Watts; L Illum, Drug Dev. Indu. Pharm., 1997, 23, 893-913.
[25]. E Mathiowitz ; JS Jacob ; YS Jong; GP Carino, Nature, 1997, 386, 410-414.
[26]. JU Kreuter; K Muller, Int. J. Pharm., 1989, 55, 39-45.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









