 About Authors:
About Authors:
*Tarun Patel, Prof. Dr. Vipin Kukkar, Krunal Parik
Seth G.L. Bihani S.D. College of Technical Education,
Institute of Pharmaceutical Sciences and Drug Research,
Sri Ganganagar, Rajasthan, INDIA
*tarunpatel35@gmail.com
ABSTRACT:
In pharma industry Packaging and Labelling plays very important role for improvements of attraction to human beings. So by improving our packaging and labeling style we can easily improve our product market value. Green packaging is also an alternative to make packaging more environmental friendly which would not affect the nature in any way. The most desirable solution is “use less, discard less, save more, reuse more”In this review article we discuss briefly about the requirement of packaging and labeling control of product according to different GMPs.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1510
INRODUCTION
The pharma packaging industry in India is still n a nascent stage. “First impression is the last impression; packaging plays a very important role in creation that impression for any product. But when it comes to pharmaceuticals, it has to go beyond looks”. (Trend in pharma packaging, the pharma review, abstracted by international pharmaceutical abstracts and chemical abstracts) The way you package and label your product is important. First, packaging protects it from physical, chemical and microbiological invasion. The package also provides a medium for presenting advertising messages and other important information to the consumer. And finally, the package is one of the greatest influences on a consumer’s decision to try your product.
Packaging is a combination of science, art and technology. Considering the growing demand of packaging in the consumer market, it is observed that most of the consumer products manufacturing companies are demanding packaging professionals for procuring consistent qualitative packaging materials in optimum cost. In addition, it is also observed that the purchase and marketing departments various companies who deal with the packaging material require packaging experts. However, experts maintain that while investing and innovation in packaging, some key hallmarks have to be taken care of.
Packaging also makes a bridge between production and marketing. The thumb rule is applicable to pharmaceuticals as much any other product because packaging of drugs needs to convey that feel good factor to a patient who needs that drug to be cured. However, packaging is also used to disseminate important information regarding the drug like its contents or chemical formula, usage, storage, dosage, precautions related the drug usage, dates of manufacturing and expiry, batch number etc.(Trend in pharma packaging, the pharma review, abstracted by international pharmaceutical abstracts and chemical abstracts)
[adsense:468x15:2204050025]
GENERAL GUIDELINES
Ideally, the samples should be taken from trial batches. Samples should be assembled in complete packaging materials. These studies should be made by storage testing. These studies will indicate effect of packaging material on the product & effect of product on the immediate packaging material, if the product & packaging material is finally decided. If not compatible, other packaging materials are tried.
After the final decision on packaging material has been taken, the product is subjected to shelf life studies to find out the loss of volatile material from the product, perfume y smelling & volatile material by weighing.
Finally other tests performed- in-use tests as well as laboratory tests like pour ability test, viscosity softening point, color, pH etc.
The FDA Guidance for Industry, Container Closure Systems for Packaging Human Drugs and Biologics, addresses the review and evaluation of packaging requirements. According to this document, each New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) should contain enough information to demonstrate that a proposed container closure system and its componentsare suitable for its intended use.
The type and extent of information required will depend on the dosage form and route of administration. Qualification and quality review is applied to packaging materials and to the actual dosage form. Packaging suitability is based on four attributes: protection, safety, compatibility and performance (function and/or drug delivery). For inject able dosage forms, the document outlines the tests required to show that interaction is not a problem. Associated components, such as those used only at the time a dosage is administered, self-adhesive labels and secondary packaging materials are also included in the review process.
Inhalation and injection drug products have the highest requirements. There are product-specific draft guidelines for Metered Dose Inhalers (MDI), Dry Powder Inhalers (DPI), nasal sprays and inhalation solutions, suspensions and spray drug products. The identity and concentration of leachables in inhalation and nasal drug products must be monitored throughout the dosage form's shelf life, since the product consists of the dosage form and container closure system.
Extractable and Leachable in Primary Container/Closures Systems as well as other packaging components have the potential to interact with the dosage form. Factors that must be considered in evaluating container closure systems are: materials of construction of the container/closure system, surface treatments and/or processing aids, dosage form active ingredient and excipients, sterilization and/ or other related processing and storage conditions.
The presence of extractable is determined through artificial means. An extractable is a chemical species that can be released from a container or closure material of construction that has the potential for contaminating the dosage form. Under certain exaggerated solvent, temperature and time conditions, an extractable may be generated through an interaction with the closure system.
Extractable testing studies are recommended even if containers or closures meet compendial suitability tests. Extensive testing for extractables should be performed as part of the qualification of the container/closure components. Testing under stressed conditions should demonstrate that the extractable profile is acceptable for the specific dosage form and that levels observed will not be approached or exceeded during the shelf life of the drug product.
A leachable is a chemical species that has migrated from packaging or other components into the dosage form under normal conditions of use or during stability studies. Leachables are substances identified in a defined laboratory regimen by simulating use conditions. The industry is focused on potential problems associated with extraction of chemicals from packaging materials into drug product. Leachables are a subset of extractables.(Sharma P.P.)
REGULATIONS IN PHARMA PACKAGING
In India there are two major government agencies responsible for drug regulation and control:
1. The drug controller general of India (DCGI)
2. The state food and drug a dministrations (FDAs)
The guidelines divides the parenteral (glass or plastic) and non-parentral (glass, plastic or metal) along with pressurized containers and bulk containers. Closures and liners are also given prominence.
The FDA does not approve containers as such, but only the materials used in the container. A list of substances considered “generally recognized as sage” (GRAS) has been published by the FDA. In the opinion of qualified experts they are safe under specified conditions, assuming they are of good commercial quality.
A material that is not included under FRAS or prior sanction, and is intended to be used with food, must be tested by the manufacturer, and the data must be submitted to the FDA.
The FDA has published regulations (part 133) that implement the current good manufacturing practice requirements of section 501(a) of the act Numerous guidance (see Table A below) mention the appropriate evaluation of packaging components. This guidance recommends that the safety and compatibility of the dosage form with the primary container closure system be established early in the development process. Specific focus is on the potential for drug/biologic interaction with the container or closure because of leaching or absorption.(Jenkins A. Wilmer)
|
||||||||||
Industry- based working groups have been established to assess extractable concerns and other scientific issues. The Product Quality Research Institute (PQRI) was established to conduct research that generates scientific information to support the development of regulatory policy. PQRI is driven by its member organizations which include (but are not limited to) the American Association of Pharmaceutical Scientists (AAPS), the Pharmaceutical Research and Manufacturers Association (PhRMA), the Generic Pharmaceutical Association (GPhA), the Parenteral Drug Association (PDA) and the U.S. Food and Drug Administration Center for Drug Evaluation and Research (FDA/CDER). PQRI is a nonprofit foundation that serves as a vehicle for FDA, industry and academia to collaborate on key issues in pharmaceutical product quality through research and expert analysis. Currently, PQRI's working group for leachables and extractables is attempting to better define and clarify analytical and toxicological issues relating to these key areas.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PACKAGING AND LABELLING CONTROL ACCORDING TO GMP GUIDELINE
PACKAGING MATERIALS
1. The purchase, handling and control of primary and printed packaging materials should be accorded attention similar to that given to starting materials.
2. Particular attention should be paid to printed materials. They should be stored in adequately secure conditions such as to exclude unauthorised access. Cut labels and other loose printed materials should be stored and transported in separate closed containers so as to avoid mix-ups. Packaging materials should be issued for use only by authorised personnel following an approved and documented procedure.
3. Each delivery or batch of printed or primary packaging material should be given a specific reference number or identification mark.
4. Outdated or obsolete primary packaging material or printed packaging material should be destroyed and this disposal recorded.
PACKAGING OPERATIONS
1. When setting up a programme for the packaging operations, particular attention should be given to minimizing the risk of cross-contamination, mix-ups or substitutions. Different products should not be packaged in close proximity unless there is physical segregation.
2. Before packaging operations are begun, steps should be taken to ensure that the work area, packaging lines, printing machines and other equipment are clean and free from any products, materials or documents previously used, if these are not required for the current operation. The line-clearance should be performed according to an appropriate check-list.
3. The name and batch number of the product being handled should be displayed at each packaging station or line.
4. All products and packaging materials to be used should be checked on delivery to the packaging department for quantity, identity and conformity with the Packaging Instructions.
5. Containers for filling should be clean before filling. Attention should be given to avoiding and removing any contaminants such as glass fragments and metal particles.
6. Normally, filling and sealing should be followed as quickly as possible by labelling. If it is not the case, appropriate procedures should be applied to ensure that no mix-ups or mislabelling can occur.
PACKAGING AND LABELLING ACCORDING TO OPPI GUIDELINE
a. Objective
To document a procedure for receipt, identification, storage, handling, sampling, examination and/or testing of labeling and packaging of materials which defines:
· material examination and uses control
· Labeling control and issuance measures including quality control inspections
· Packaging and labeling operation
b. Scope
Packaging and labeling controls are very essential for medicinal product ensuring fitness for their intended use. Error can affect the quality of packs. Particular attention should be given to:
· Minimizing the risk of cross contamination
· Mix up or substitution
· Different product should not be packed in close proximity unless there is physical segregation(OPPI guideline)
REQUIREMENT FOR PACKAGING AND LABELLING CONTROL
a. Material examination and usage control
1. Any labeling or packaging material should be release or rejected based on return specific requirement.
2. Records shall be maintain for each shipment received of each different labeling packaging material indicating receipt, examination or testing and whether accepted or rejected.
3. Labels and labeling material for each different drug product, strength dosage form shall be stored separately with suitable identification. Access to the storage area should be limited to authorized personnel.
4. Obsolete, out dated labels foils, cartons and other packaging material should be rejected and then destroyed. Necessary documents/records should be maintained.
5. Printing devices on or associated with manufacturing lines used to imprint upon the drug product until label, carton or case shall be monitored to assure that all imprinting conforms to the print specification.(OPPI guideline)
b. Labeling control and issuance measures
For a pharmaceutical company labeling material include printed cartoon, boxes, tins, printed labels, laminates, and any other descriptive material. eg, product insert/leaflet
1. Strick control should be exercised or material issued for used in drug product labeling operations. Error of this stage can some time lead to serious consequences. Miss labeling is one of the most frequent causes of product hazards and product recall. It is there for necessary to control the receipt, testing and issuance of labeling materials. Even the purchase, handling and control of primary and printed packing materials should be accorded attendance similar to that give to starting material.
2. Particular attention should be paid to printed materials. They should be stored in an adequately squared condition saw as to excluded unauthorized access. Cut label and other loose printed material should stored and transported in separate close containers so as to avoid mix up. Packaging material should be issued for use only by authorized personnel, following in approved and documented procedure.
3. Responsibility for the storage and issue of such materials should at all times, be assigned to a person designated for this purpose by the factory management.
4. All labeling materials should be coded in such as manner that the code reveals the material and indicates that the material is the one currently in use. As a corollary, it must be ensured that when there is any change in the label copy matter, the code number is suitably amended.
5. Procedure should be written describing in sufficient detail the control procedure employed for the issuance of labeling and issuance recorded.
6. On-line rejected primary packaging material or printed material should be destroyed and this disposal recorded. Excess leftover printed materials with batch details also should be destroyed to avoid mix-up.
7. Labelling materials issued for a batch shall be carefully examine for identity and conformity to the labeling specified in the master or batch production records.
8. Procedure shall be draw and implemented to reconcile the quantities of labeling received, issued, used and shall require evolution of discrepancies found between the quality of drug product packed and quantity of labeling issued when such discrepancies are outside narrow present limits based on historical operating data.
9. Lebelling returns to the stores shall be maintained and stored in a manner to prevent mix-ups and proper identification.
10. Labelling materials should be so design and printed that there is a marked differentiation between labels, cartons etc. of different product and different dosage form/strengths of the same product. This can be achieved by using different shapes, different colours and different sizes.
11. Gang printing of labels should be replaced with on-line printing to extent possible. However where inevitable, the pharmaceutical manufacturer should educate printer on the steps that should be taken to prevent mix-up, on limiting the numbers and types of labels which may be gang printet on the sheet, on segregating different printing operation and so on.
12. All labeling materials should be held under quarantine, until cleared by Quality Control.
13. Quality Control should checked among other thing like dimension, colour etc. the accuracy of all printed details in order to ensure that the copy matter is in accordance with the current statutory and in company requirements of labelling. Printed matter and colour of all packaging materials should be checked against approved specimens, which match with approved artwork that has been duly sign and dated by quality control, purchase, packaging materials development departments. For control of variation in colour, the vendor should provide shade cards should be approved standard, light and dark shades. There shade cards should be approved by quality control, and it must be ensured that all supplies are within the acceptable rang.
14. Overprinting of Batch coding details etc. when done must be authorized.
15. To prevent labeling and packing errors, a know and recorded number of properly coded labeling materials should be issued with proper security against signed packaging order that indicates quantity and type of labeling materials required for packaging operation.(OPPI guideline)
c. Packaging and labeling operation
It is of almost importance to ensure that correct labels, labeling and packaging materials are used for drug product. The entire operation should be well define via return procedure and followed in totality. Particular attention should be given to ensure that different products are not close in close proximity unless there is physical segregation. The written procedure should include following features, which will prevent mix-ups and cross-contamination:
1. Before packaging operation begin, steps should be taken to ensure that the work area, packaging lines, printing machines and other equipments are clean and free from other products, materials and documents previously used, if there are not required for current operation. A line clearance should be perform according to an appropriate checked list and documents.
2. Identification of drug product with a lot or control number that permits determination of the history of manufacture and control of the batch.
3. The name and lot number of product being handled should be display at each packaging station or line.
4. All products, labels, labeling and packaging materials should be checked on delivery to the packaging department of quality, identity and conformity with the packaging instruction.
5. Examination of packaging and labeling materials for suitability and correctness before start-up of packaging operation and documentation of such examination in the batch production record. Attention should be paid to printing by head which should be rechecked at regular intervals. Specimen copy of label should be kept with each batch record. All stereos used for on-line printing should be thoroughly checked for correctness and legibility of printed matter. Used stereos should be collected and suitable destroyed after completion of the batch.
6. Normally, filling and sealing should be clean before filling. Attention should be given for avoiding and removing contaminants if any, such as glass fragments and metal particles.
7. Normally filling and sealing should be followed immediately by labeling, if it is not the case appropriate procedure should be implemented to ensure that no mi-ups and mislabeling can occur.
8. Special care should be taken when using cut labels and when overprinting is carried out off-line. Roll-feed labels are normally preferred for cut labels, in helping avoid mix-ups and ensuring accountability.
9. Periodic validation should be conducted to ensure that electronic electronic code readers, label counter or similar devices operating correctly. Necessary in-process checked and record should be maintained.
10. Printed and embossed information on packaging materials should be distinct and resistant to fading and erasing.
11. On-line control of the product during packaging include at least checking following:
* General appearance of the packages
* Whether the packages are complete and the count is as per specification
* Whether correct products and packaging materials are used
* Whether overprinting if any, is corrected
* Correct functioning of line controls.
12. Sample taken away from packaging line should not be returned. They should where appropriate, be destroyed (e.g. Leak tested strips).
13. Product lots which have been involved in special events (like rework/ re-inspection in part or whole) should be re-introdused into process only after additional inspection and approval is carried out by appropriate authorised personnel. Detail record of any such deviation should be maintained.
14.Any significant and unusual discrepancy observed during reconciliation of the amount of bulk product and printed packaging materials and the numbers of units products produced should be investigated and satisfactorily accounted for before final release of the packaged product.
15. On completion of a packaging operation, any unused batch coded packaging material should be destroyed and the destruction recorded. A documented procedures should be followed if uncoded printed material is returned to stock.
16. Usage of packaging material during rework should be recorded separately whenever necessary.(OPPI guideline)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
REQUIREMENTS FOR TAMPER-EVIDENT PACKAGING
a. Objective
To establish uniform requirements or temper evident packing of drug products that will improve security of drug packing and help assure the safety and effectiveness of the drug product.
Definition:
A. Temper evident package is one having and indicator or barrier to enter which if breached or missing can reasonably the be expected to provide visible evidence to consumer that tempering has occur.
b. General Requirement
1. Each manufacture and packer who packes drug product for retail sale shall packed the product in a temper evident package. Specially for drug intended for internal consumption.
2. To reduce the likely hood of substitution of a temper evident feature after tempering the indicator or barrier to entry, it is necessary that device used be distinctive in design (i.e. can not be duplicated with commonly available materials or processes) or by the use of an identifying characteristic.
3. A temper evident package may involved and immediate container and closure and or secondary container or cartoon system or any combination of systems intended to provide a visual identification package integrity.
4. Temper evident feature shall be design to and shall remain intact during manufacture, distribution and retail display.(Leon Lachman )
c. Labelling
1. Each retail package of drug product is required to bear a statement that is prominently place so that consumers are alerted to specific temper evident features of the package.
2. The labeling statement is also required to be so placed that it will be unaffected, if the temper- evident feature the packages breached or missing.
3. A particular characteristic of temper evident nature is required to be referred to in the labeling statement. i.e. “For your protection, this bottle has an imprinted seal around the neck”(OPPI guideline)
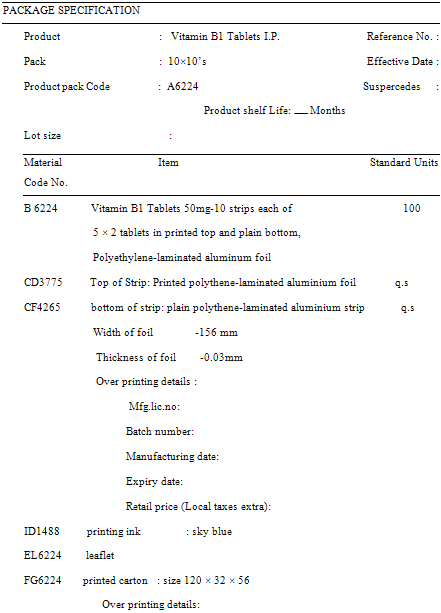
PACKAGING SPECIFICATION
a. Objective
Standards for the final package of drug products are as important as standards for the product themselves. It is there for essential that correct packing material, correct label and labeling is used for every product. The packing specification are prepared on the basis of stability studies done on the product packes during the development stage and also on the machine capability trials carried out prior to routine manufacture.
b. General Guidelines
Packaging specifications are the detail requirements for completing a pack. Each specification must have a specific reference to unable a planning staff to quote the specification while scheduling the orders.
1. Master packing instruction should be created for every product in every pack in which it is sold. These document should least, among other things, a list of components to be used for a stated container or package in the form of packaging specification or packing profile.
2. For every product package all the component (packaging material) should be listed along with their code or reference number which will related them to their specification. The quantities, sizes and types of packaging material, over printing and other details should be stated in the packaging specification.
3. Where practicably is specimen or facsimile or the relevant printed packaging material should be attached to the packaging specification.
4. Special precaution should be taken during packing operations. i.e. The packaging of moisture sensitive tablet will require specific humidity and temperature controls.
5. Printed matter should be as per approved art work or approved copy.
6. Specimens of package specification are given in the appendices. Please note that the detail mention below serve only as examples and not recommended specifications for any materials. In reality they may vary with manufactures/material/products.(OPPI guideline)



LINE CLEARANCE
a.Objective
An important requirement of GMP is that the packaging and labeling facilities are inspected before use to assure that all drug products have been removed from previous operation and that packaging and labealing materials not suitable for subsequent operation have also been removed. It is also stipulated that the results of inspection are documented in the batch production records.
b. Procedure
1. The filling, packaging line and the area immediately closed to it should be free from all filled / package products as well as all the packaging and labeling material from the previous filling / packaging operation.
2. Rejects on the line such as in adequately filled bottles, broken or damaged packs, strips with empty pouches and so on should also be removed and destroyed.
3. Spillages on the line and the immediate areas should be mopped up and / or cleared.
4. Particular attention should be paid to left over labels and cartons with over printed lot details from the previous rune, especially when a new batch of the same product is to be packed on the lines.
5. It should be certified that the packaging equipment has been properly cleaned and a tag (preferably green coloured and marked ‘CLEANED’) indicating the status should be attached to it. The tag should also indicate the date of cleaning and the name of product for which the equipment was last used and should be signed by the supervisor.
6. If the equipment has been idle even for a short period of time before use, it should be re inspected for cleanliness and re cleaned before use, if required. The green tag should indicate the date of the new cleaning and should be initialed by the supervision.
7. Information regarding cleaning of major packaging equipment should be recorder in the, ‘Equipment Cleaning and Use Log’ of the packaging department.
8. If the line clearance is satisfactory, the relevant portion of the filling/ packaging order should be filled and initialed by supervisor.
9. Filling or packaging of a batch should not commence until the line clearance has been initialed by the supervisor on the filling / packaging order.
10. Where the filling /packaging has been interrupted, a new line clearance check has to be made. In such cases, all the materials should be returned, under close supervision to their respective identified containers until the new clearance has been made.(OPPI guideline)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PACKAGING INSTRUCTIONS AND REQUIREMENTS ON FINISHED PRODUCT INSPECTION
a. Packaging Instructions
The objective of this section is to have written documents of the packaging operation (also known as Packaging Direction Record). In short, details about the packaging operation, various materials used in packaging operations and critical packaging parameters are documented. For example / if an ointment is being packed, it is advisable to record the temperature at which the packaging operation was carried out. This will help in keeping not only the product quality uniform within the batch, but also between batches. All this are covered step wise in this section.
If any in process checks are carried out, it must be recoded. e.g, weight / fill volume data, filling temperature etc. this can be either in a separate register or as an attachment to the packaging records.
It is likely that there may be many variation in packaging items required for each product, depending on the pack size. It is there for essential that clearly define packaging instruction must be available to the packaging line supervision and engineering staff / operator who are setting up the line. The line supervisor will required the Approved Packaging Specifications, along with specific batch details of the products. The engineering staff / operator will required the line setup instructions.
Packaging instruction should be distinguished from packaging orders. Packaging instruction provide a detail description of all sequences which are to occur in packaging labeling so that there is minimal change of error. Packaging orders determine specific operational and quantitative requirements for each packaging cycle to be carried out in accordance with the corresponding packaging instruction. It is there for, necessary to have formally authorized packaging instructions for each product, pack size and type. There should normally include the following:
1. Name of the product;
2. Description of its pharmaceutical form, strength and, where applicable, method of application;
3. The pack size expressed in terms of the number, weight or volume of the product in the ?nal container;
4. A complete list of all the packaging materials required for a standard batch size, including quantities, sizes and types, with the code or reference number relating to the speci?cations for each packaging material;
5. Where appropriate, an example or reproduction of the relevant printed packaging materials and specimens, indicating where the batch number and expiry date of the product have been marked;
6. Special precautions to be observed, including a careful examination of the packaging area and equipment in order to ascertain the line clearance before and after packaging operations;
7. A description of the packaging operation, including any signi?cant subsidiary operations, and equipment to be used;
8. Details of in-process controls with instructions for sampling and acceptance limits.
9. An approved specimen or facsimile of relevant printed packaging material(where practicable)
10. Details of any required preparation of packaging materials (e.g. sterilizing, overprinting of batch details and retail price, inspection of empty bottle for liquids etc.)
11. Special characteristics to be measured and controlled during filling, packaging and labeling (e.g. volume of fills, clarity, homogeneity of suspension, humidity control, nitrogen blanketing during liquid filling etc.)
12. Details of outer packaging operation including the relevant labeling requirements.
13. Personnel on an assembly line should be adequate to ensure that each and every unit of finished product is inspected for defected.
14. Packaging order are specified for a packaging cycle involving one batch of one product. They normally include the following:
a) The name of product.
b) The code number of the product in the packaged form
c) The dosage form and strength of the product.
d) The batch number of the bulk product being packaged.
e) The packaging “lot” or “control” number assigned to the packaging operation, where a batch of a product is filled in more than one lot (in such cases reference should be made to the batch number means some identification number for the specific quantity of packaging material received at the site.
f) The quantity of final packs required and the amount of packaging materials required according to the relevant to Pack Specification.
g) The relevant overprinting details such as batch number, date of manufacture, expiry date, retail price or Physician Sample not for Sale etc.
h) Any special instruction applicable for a particular operation including specific labeling requirements on the outer shipment packaged.(OPPI guideline)
Requirements of finished product inspection
1. Packaged and labeled product should be examined during packaging operation to provide assurance that containers packaged in the lot have the correct label.
2. A representative sample of units shall be collected throughout the packaging operation and shall be visually examined for correct labeling.
3. Results of these examination shall be recorded in the batch production or control record.(OPPI guideline).
PACKAGING AND LABELING CONTROLS ACCORDING TO USFDA GUIDELINES
a. Materials Examination and Usage
Medical gases are subject to the requirements in Materials examination and usage criteria. There must be written procedures describing in sufficient detail the receipt, identification, storage, handling, sampling, and examination of labeling and packaging materials and these written procedures must be followed. Labeling and packaging materials must be representatively sampled, and examined or tested upon receipt and before use in packaging or labeling of a medical gas.
Records must be maintained for each shipment received of each different labeling indicating receipt, examination, and whether accepted or rejected.
Labels for each different medical gas must be stored separately with suitable identification.
Access to the storage area must be limited to authorized personnel.
Obsolete and outdated labels must be destroyed.
If cut labeling is used, labeling operations must include one of the following special control.
Procedures
1. Dedication of labeling lines to each different strength of each different medical gas
2. Use of appropriate electronic or electromechanical equipment to conduct a 100 percent examination for correct labeling during or after completion of finishing operations
3. Use of visual inspection to conduct a 100 percent examination for correct labeling during or after completion of finishing operations for hand-applied labeling. Such examination must be performed by one person and independently verified by a second person.
Upon receipt from the printer, labels would be counted to verify the quantity received and would be examined to ensure correctness when compared against the master label.
We recommend that labels be locked in a secure area with access limited to authorized personnel. Different medical gas labels would be stored separately. We recommend that industrial labels be stored in a separate area.
It is industry practice to apply labels by hand, therefore, we recommend a second person verify the correctness of the label and document the verification. In light of recent deaths and injuries, this examination is critical to ensure that the correct label has been applied to a container of medical gas.(USFDA guideline)
b. Labeling Control
Medical gases are subject to the requirements in Labeling issuance. Strict control must be exercised over labeling issued for use in medical gas labeling operations.
Labeling materials issued for a batch must be carefully examined for identity and conformity to the labeling specified in the master or batch production records.
Procedures must be used to reconcile the quantities of labeling issued, used, and returned, and must require evaluation of discrepancies found between the quantity of drug product finished and the quantity of labeling issued if the discrepancies are outside narrow preset limits based on historical operating data. However, this paragraph does not apply to the 360-degree wrap-around label that is applied to large cryogenic containers.
The Agency recommends that all labels be issued by authorized personnel only. Before release of issued labels to an employee, we recommend a representative label be checked against the master label to ensure correctness.(Leon Lachman)
c. Packaging and Labeling Operations
Medical gases are subject to the requirements in Packaging and labeling operations.
There must be written procedures designed to ensure that correct labels and labeling are used for medical gases; such written procedures must be followed. These procedures must incorporate
the following features:
1. Prevention of mix-ups and cross contamination by physical or spatial separation from operations on other medical gases
2. Identification of the medical gas with a lot or control number that permits determination of the history of the manufacture and control of the batch
3. Examination of labeling materials for suitability and correctness before packaging operations, and documentation of such examination in the batch production record
We recommend manufacturers consider each batch of medical gas a separate entity with unique filling procedures to help ensure that the batch is uniform and consistent. Assigning a single lot number to an entire day's production is not appropriate. Each manifold filling sequence; each uninterrupted filling sequence; and each filled cryogenic container, storage tank, and trailer would be considered a new lot and be assigned a unique lot number.
In addition, we recommend each large cryogenic container containing liquid oxygen for delivery to patients at home, whether portable or permanently mounted in a van or a truck, be considered a lot and be assigned a unique lot number. Cryogenic home containers filled at a patient's home do not need a lot number. However, we recommend that cryogenic home containers filled onsite or by a third party in advance for future delivery be given a lot number.
For safety reasons, we recommend each medical gas container bear only one drug label containing the appropriate information. Do not place a current label on top of an obsolete label.
In accordance with 502(b)(2) of the Act, all medical gas cylinders and cryogenic containers must bear a label with an accurate statement of the net contents. We recommend that the net contents appear on the body label or shoulder label and not on (1) a removable tag, (2) a certificate of analysis, or (3) a small separate sticker.
If a medical gas company sells medical oxygen to emergency medical services for emergency use, the label would contain the statement:
For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx Only.
FDA would not prohibit the sale of medical oxygen with this labeling to emergency medical services (see Glossary for definition of an EMS) without a prescription.
We recommend the labeling for large permanently mounted containers, trailers, and rail cars bear a statement consisting of “Name of the Medical Gas, Refrigerated Liquid USP or NF,” such as "Oxygen Refrigerated Liquid USP."
The Agency recommends the use of a 360-degree wrap-around label to identify medical gases in large cryogenic containers.(USFDA guideline, Shah D.H.)
D. Drug Product Inspection
Medical gases are subject to the requirements in § 211.134 – Drug product inspection.Labeled products must be examined during finishing operations to provide assurance that containers in the lot have the correct label.
A representative sample of units must be collected at the completion of finishing operations and must be visually examined for correct labeling.Results of these examinations must be recorded in the batch production or control records.
Only one medical gas label would appear on a cylinder or container, and the manufacturer of the medical gas would apply the label in accordance with section 502(b) of the act.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org









