ABOUT AUTHORS:
MHG Dehgan, Satapathy Asis Amitav*
Y.B.Chavan College of Pharmacy,
Dr. Rafiq Zakaria Campus,
Auranagbad-431001 (MS),
*asish.apharma@gmail.com
ABSTRACT
Nasal route for the administration of drugs is used as an alternative route for the systemic availability of drugs restricted to intravenous administration. This is due to the large surface area, porous endothelial membrane, high total blood flow, the avoidance of first-pass metabolism, and ready accessibility. The nasal administration of drugs, including many biotechnological compounds like hormones, vaccines, peptide and protein drugs to give enhanced bioavailability. Many drug delivery methods for nasal availability of liquid, semisolid and solid formulation are investigated to avail the drugs to treat most CNS diseases (i.e., Parkinson’s disease, Alzheimer’s disease, Migraine attack) because it requires rapid and/or specific targeted delivery of drugs to the brain.
In this review we discuss some factors affecting nasal absorption, bio-availability barriers, and strategies to improve nasal absorption, new developments in nasal dosage form design and applications of nasal drug delivery system and the effects of microspheres, liposomes and other bioadhesive drug delivery systems on nasal drug absorption.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1278
INTRODUCTION
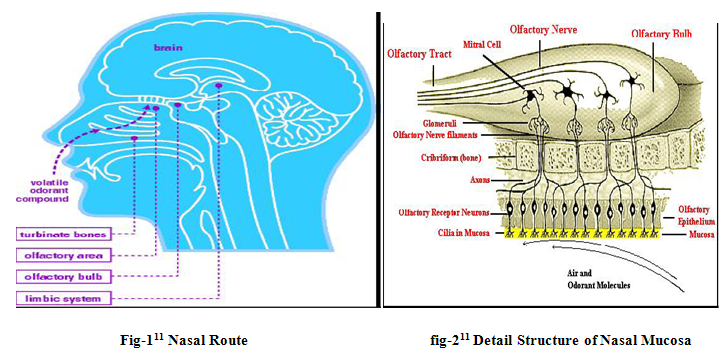
Nasal drug delivery system which has been practiced for thousands of years, has been given a new ray of life. Nasal therapy, is one of the recognized form of treatment in the Ayurvedic systems of Indian medicine, it is also called “NASAYA KARMA” [1]. It is a useful drug delivery method for drugs that are active in low doses and show less oral bioavailability such as proteins and peptides. One of the reasons for the low degree of absorption of peptides and proteins via the nasal route is rapid movement away from the absorption site in the nasal cavity due to the mucociliary clearance mechanism[2]. The nasal route overcomes hepatic first pass metabolism associated with the oral delivery. During the past several decades, the availability of drug via the nasal route has received maximum attention from pharmaceutical scientists and clinicians. Drug candidates ranging from small to large molecules to macromolecular proteins have been tested in various animal subjects[1]. It has been documented that nasal administration of certain hormones and steroids have resulted in a more complete absorption[3] . This indicates the potential value of the nasal route for administration of systemic medications as well as utilizing this route for local effects. Nasal routesoffers lower doses, more rapid attainment of therapeutic blood levels, quicker onset of pharmacological activity fewer side effects, high total blood flow per cm3, porous endothelial membrane is easily accessible, and drug is delivered directly to the brain along the olfactory nerves [4-6].However the primary function of the nose is olfaction, it heats and humidifies inspired air and also filters airborne particulates[7]. Consequently, the nose functions as a protective system against foreign material[8]. There are three distinct functional zones in the nasal cavity, namely: vestibular, olfactory, and respiratory areas. The vestibular area serves as a baffle system; it functions as a filter of airborne particles[9].The olfactory epithelium is capable ofmetabolizing drugs. The respiratory mucosa is the region where drug absorption is optimal[10].To optimize nasal administration, bioadhesive hydrogels, bioadhesive microspheres (dextran, albumin and degradable starch) and liposomes have been studied.
FACTORS INFLUENCING NASAL DRUG ABSORPTION
There are various factors that affect the systemic bioavailability of drugs that are administered through the nasal route. The factors can be affecting to the physiochemical properties of the drugs, the anatomical and physiological properties of the nasal cavity and the type and characteristics of selected nasal drugs delivery system. These factors play key role for most of the drugs in order to reach therapeutically effective blood levels after nasal administration. The factors influencing nasal drug absorption are described as follows.
[adsense:468x15:2204050025]
1) Physiochemical properties of drug.
* Lipophilic-hydrophilic balance.
* Enzymatic degradation in nasal cavity
* Molecular size.
2) Delivery Effect
* Delivery effects
* Formulation (Concentration, pH, osmolarity)
* Drugs distribution and deposition.
* Viscosity
3) Nasal Effect
* Mucociliary clearance
* Cold, rhinitis.
* Membrane permeability.
* Environmental pH
1) Physiochemical properties of drug
Lipophilic-hydrophilic balance
The HLB nature of the drugs affects the absorption process. By increasing lipophilicity, the permeation of the compound normally increases through nasal mucosa. Although the nasal mucosa was found to have some hydrophilic character, it appears that these mucosae are primarily lipophilic in nature and the lipid domain plays an important role in the barrier function of these membranes. Lipophilic drugs like naloxone, buprenorphine, testosterone and 17a-ethinyl- oestradiol are almost completely absorbed when administered intranasal route [11-12].
Enzymatic degradation in nasal cavity
Drugs like peptides and proteins are having low bio-availability across the nasal cavity, so these drugs may have possibility to undergo enzymatic degradation of the drug molecule in the lumen of the nasal cavity or during passage through the epithelial barrier. These both sites are having exo-peptidases and endo-peptidases, exo-peptidases are mono-aminopeptidases and di-aminopeptidases. These are having capability to cleave peptides at their N and C termini and endo-peptidases such as serine and cysteine, which can attack internal peptide bonds [13].
Molecular size
The molecular size of the drug influence absorption of the drug through the nasal route. The lipophilic drugs have direct relationship between the MW and drug permeation whereas water soluble compounds depict an inverse relationship. The rate of permeation is highly sensitive to molecular size for compounds with MW ≥ 300 Daltons [14].
2) Delivery effect factors
Factors that affect the delivery of drug across nasal mucosa such as surfactants, dose pH, osmolarity, viscosity, particle size and nasal clearance, drug structure can be used to advantage to improve absorption.
Formulation (Osmolarity, , pH, Concentration)
The osmolarity of the dosage form affects the nasal absorption of the drug; it was studied in the rats by using model drug. The sodium chloride concentration of the formulation affects the nasal absorption. The maximum absorption was achieved by 0.462 M sodium chloride concentration; the higher concentration not only causes increased bioavailability but also leads to the toxicity to the nasal epithelium [15].
The pH of the formulation and nasal surface, can affect a drug’s permeation. To avoid nasal irritation, the pH of the nasal formulation should be adjusted to 4.5–6.5 because lysozyme is found in nasal secretions, which is responsible for destroying certain bacteria at acidic pH. Under alkaline conditions, lysozyme is inactivated and the tissue is susceptible to microbial infection. In addition to avoiding irritation, it results in obtaining efficient drug permeation and prevents the growth of bacteria [16].
Concentration gradient plays very important role in the absorption / permeation process of drug through the nasal membrane due to nasal mucosal damage. Examples for this are nasal absorption of L-Tyrosine was shown to increase with drug concentration in nasal perfusion experiments. Another is absorption of salicylic acid was found to decline with concentration. This decline is likely due to nasal mucosa damage by the permanent [17].
Drugs distribution and deposition
The drug distribution in the nasal cavity is one of the important factors, which affect the efficiency of nasal absorption. The mode of drug administration could effect the distribution of drug in nasal cavity, which in turn will determine the absorption efficiency of a drug. The absorption and bioavailability of the nasal dosage forms mainly depends on the site of disposition. The anterior portion of the nose provides a prolonged nasal residential time for disposition of formulation, it enhances the absorption of the drug. And the posterior chamber of nasal cavity will use for the deposition of dosage form; it is eliminated by the mucociliary clearance process and hence shows low bioavailability [18]. The site of disposition and distribution of the dosage forms are mainly depends on delivery device, mode of administration, physicochemical properties of drug molecule.
Viscosity
A higher viscosity of the formulation increases contact time between the drug and the nasal mucosa thereby increasing the time for permeation. At the same time, highly viscous formulations interfere with the normal functions like ciliary beating or mucociliary clearance and thus alter the permeability of drugs.
3) Nasal effect factors
Mucociliary clearance
Particles entrapped in the mucus layer are transported with it thus, effectively cleared from the nasal cavity. The combined action of mucus layer and cilia is called mucociliary clearance. This is an important, nonspecific physiological defence mechanism of the respiratory tract to protect the body against noxious inhaled materials[11]. The normal mucociliary transit time in humans has been reported to be 12 to 15 minutes[[19].The factors that affect mucocilliary clearance include physiological factors (age, sex, posture, sleep[20],exercise [21],common environmental pollutants (sulphur dioxide and sulphuric acid, nitrogen dioxide, ozone, hairspray and tobacco smoke[22], diseases (immotile cilia syndrome, primary ciliary dyskinesia- Kartagener.s syndrome, asthma, bronchiectasis, chronic bronchitis, cystic fibrosis, acute respiratory tract infection[23] and drugs[24] and additives [25].
Cold, rhinitis
Rhinitis is a most frequently associated common disease, it influence the bioavailability of the drug. It is mainly classified into allergic rhinitis and common, the symptoms are hyper secretion, itching and sneezing mainly caused by the viruses, bacteria or irritants. Allergic rhinitis is the allergic airway disease, which affects 10% of population. It is caused by chronic or acute inflammation of the mucous membrane of the nose. These conditions affect the absorption of drug through the mucus membrane due the inflammation
Membrane permeability
Nasal membrane permeability is the most important factor, which affect the absorption of the drug through the nasal route. The water soluble drugs and particularly large molecular weight drugs like peptides and proteins are having the low membrane permeability. So the compounds like peptides and proteins are main-ly absorbed through the endocytotic transport process in low amounts [26]. Water-soluble high molecular weight drugs cross the nasal mucosa mainly by passive diffusion through the aqueous pores (i.e. tight junctions).
Environmental pH
The environmental pH plays an important role in the efficiency of nasal drug absorption. Small water-soluble compounds such as benzoic acid, salicylic acid, and alkaloid acid show that their nasal absorption in rat occurred to the greatest extent at those pH values where these compounds are in the nonionised form. However, at pH values where these compounds are partially ionized, substantial absorption was found. This means that the nonionised lipophilic form crosses the nasal epithelial barrier via transcellular route, whe-reas the more lipophilic ionized form passes through the aqueous paracellular route [27].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PROFILE OF AN ‘IDEAL’ DRUG CANDIDATE FOR NASAL DELIVERY
Ø Appropriate aqueous solubility to provide the desired dose in a 25–150 µl volume of formulation administered per nostril; providing the therapeutic effects.
Ø Appropriate nasal absorption properties;
Ø No nasal irritation from the drug;
Ø A suitable clinical rationale for nasal dosage forms, e.g. rapid onset of action;
Ø Low dose. Generally, below 25 mg per dose;
Ø No toxic nasal metabolites;
Ø No offensive odors/aroma associated with drug;
Ø Suitable stability characteristics[28].
ADVANTAGES
The advantages of intranasal delivery are considerable:
(1) This method is Non-invasive, rapid and comfortable.
(2) Bypasses the BBB and targets the CNS, reducing systemic exposure and thus systemic side effects.
(3) Does not require any modification of the therapeutic agent being delivered.
(4) Works for a wide range of drugs. It facilitates the treatment of many neurologic and psychiatric disorders.
(5) Rich vasculature and highly permeable structure of the nasal mucosa greatly enhance drug absorption.
(6) Problem of degradation of peptide drugs is minimized up to a certain extent.
(7) Easy accessibility to blood capillaries.
(8) Avoids destruction in the gastrointestinal tract, hepatic “first pass” elimination and gut wall metabolism, allowing increased, reliable bioavailability.
(9) Direct delivery of vaccine to lymphatic tissue and induction of a secretory immune response at distant mucosal site [29].
(10) A porous endothelial basement membrane that poses no restriction to transporting the drug into general circulation.
(11) Realization of pulsatile delivery of some drugs like human growth hormone, insulin, etc., is higher with nasal drug delivery.
(13) Reduced risk of infectious disease transmission[30]
(14) Reformulation of existing drugs as nasal drug delivery products offers companies the possibility to extend the life cycle of their products[31].
LIMITATIONS
(1) Concentration achievable in different regions of the brain and spinal cord varies with each agent.
(2) Delivery is expected to decrease with increasing molecular weight of drug.
(3) Some therapeutic agents may be susceptible to partial degradation in the nasal mucosa or may cause irritation to the mucosa.
(4) Nasal congestion due to cold or allergies may interfere with this method of delivery.
(5) Frequent use of this route results in mucosal damage (e.g. infection, anosmia)[32].
(6) It could also lead to irreproducibility of the dosing regimen as a result of drainage of the solution or expulsion of the dose due to sneezing[31].
ANATOMY & PHYSIOLOGY OF NASAL CAVITY
The nasal cavity is divided into two halves by the nasal septum and extends posterior to the nasopharynx, while the most anterior part of the nasal cavity, the nasal vestibule, opens to the face through the nostril. The nasal cavity consists three main regions are nasal vestibule, olfactory region and respiratory region. The surface area in the nose can be enlarges about 150cm2 by the lateral walls of the nasal cavity includes a folded structure, it is a very high surface area compared to its small volume. This folded structure consists of three turbinates: the superior, the median and the inferior [33].The main nasal airway having the narrow passages, usually it has 1-3mm wide and these narrows structures are useful to nose to carry out its main functions.
The nasal cavity is covered with a mucous membrane which can be divided into two areas; nonolfactory and olfactory epithelium, in this non-olfactory area includes the nasal vestibule which is covered with skin-like stratified squamous epithelium cells, where as respiratory region, which has a typical airways epithelium covered with numerous microvilli, resulting in a large surface area available for drug absorption and transport [34]. In this way the mucus layer is propelled in a direction from the anterior to-wards the posterior part of the nasal cavity. The goblet cells are present in the mucus membrane which covers the nasal turbinate and the atrium; it secretes the mucus as mucus granules which are swelling in the nasal fluid to contribute to the mucus layer.
The mucus secretion is composed of about 95% water, 2 % mucin, 1% salts, 1% of other proteins such as albumin, immunoglobulins, lysozyme and lactoferrin, and b 1% lipids [35]. The mucus secretion gives immune protection against inhaled bacte-ria and viruses.It also performs a number of physiological functions. (1) It covers the mucosa, and physically and enzymatically protects it. (2) The mucus has water-holding capacity. (3) It exhibits surface electrical activity. (4) It permits efficient heat transfer. (5) It acts as adhesive and transport s particulate matter towards the naso pharynx [36].
MECHANISM OF NASAL ABSORPTION
The first step in the absorption of drug from the nasal cavity is passage through the mucus. Small, unchanged particles easily pass through this layer. However, large or charged particles may find it more difficult to cross. Mucin, the principle protein in the mucus, has the potential to bind to solutes, hindering diffusion. Additionally, structural changes in the mucus layer are possible as a result of environmental changes (i.e. pH, temperature, etc.) Subsequent to a drug’s passage through the mucus, there are several mechanisms for absorption through the mucosa. These include trans-cellular or simple diffusion across the membrane, paracellular transport via movement between cell and transcytosis by vesicle carriers [37]. Obstacles to drug absorption are potential metabolism before reaching the systemic circulation and limited residence time in the cavity. Several mechanisms have been proposed but the following two mechanisms have been considered predominantly. The first mechanism involves an aqueous route of transport, which is also known as the paracellular route. This route is slow and passive. There is an inverse log-log correlation between intranasal absorption and the molecular weight of water-soluble compounds. Poor bioavailability was observed for drugs with a molecular weight greater than 1000 Da [38].
The second mechanism involves transport through a lipoidal route that is also known as the transcellular process and is responsible for the transport of lipophilic drugs that show a rate dependency on their lipophilicity. Drugs also cross cell membranes by an active transport route via carrier-mediated means or transport through the opening of tight junctions [38].
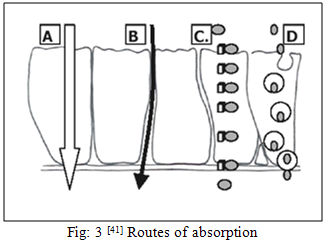
(A. Passive intracellular transport, B. Paracellular/ tight junction transport, C. Carrier mediated transcellular transport, D. Transcellular transcytosis)
Barriers for nasal drug absorption
Mucociliary clearance
Particles entrapped in the mucus layer are transported with it and thereby effectively cleared from the nasal cavity. The combined action of the mucus layer and cilia is called mucociliary clearance. This is an important, non-specific physiological defence mechanism of the respiratory tract to protect against noxious inhaled materials. Mucus traps the particles of dust, bacteria and drug substances and is transported towards the nasopharynx at a speed of 5 - 8 mm/min [39], where it is swallowed. The normal mucociliary transit time in humans has been reported to be 12 to 15 min [39].
Protective barriers
The first step in the absorption of drugs from the nasal cavity passed through the mucus. Uncharged substances with small molecular weight can easily pass through this layer. However, larger or charged particles may find it more difficult to cross. Mucin, the principal protein in the mucus, has the potential to bind to solutes, hindering diffusion. Additionally, structural changes in the mucus layer are possible as a result of environmental changes such as pH, temperature etc. The nasal membrane is a physical barrier and the mucociliary clearance is a temporal barrier to drug absorption across the nasal epithelium.
Enzymatic barrier
The role of the enzymatic barrier is to protectthe lower respiratory airways from toxic agents; the nasal mucosa contains many enzymes such as cytochrome P450-dependent monooxygenase, carboxyl esterase and amino peptidase. Although nasal delivery avoids hepatic first-pass metabolism to some extent, the nasal mucosa provides a pseudo-first-pass effect. In addition, there are various barriers in the nasal membrane for protection from the microorganisms, allergens and irritating substances from the environment that must be overcome by drugs before they can be absorbed into the systemic circulation [40].
STRATEGIES TO IMPROVE NASAL ABSORPTION
Various strategies used to improve the bioavailability of the drug in the nasal mucosa which includes
1. To enhance nasal absorption
2. To improve the nasal residence time
3. To modify drug structure to change physicochemical properties.
Any one or combination of above approaches are used for the enhancing the absorption and bioavailability of the formulations. Several methods have been used to facilitate the nasal absorption of drugs includes:
Nasal enzyme inhibitors
Nasal metabolism of drugs can be increased by using the enzyme inhibitors. Mainly for the formulation of proteins and peptide molecule development enzyme inhibitors like peptidases and proteases are used [41]. The absorption enhancers like salts and fusidic acid derivatives also shows enzyme inhibition activity to increase the absorption and bio-availability of the drug [42]. The other enzyme inhibitors commonly used for the enzymatic activity are tripsin, aprotinin, borovaline, amastatin, bestatin and boroleucin inhibitors.
Prodrug approach
Prodrug approach is mainly meant for optimizing favorable physicochemical properties such as solubility, taste, odor, stability, etc. Prodrug is usually referred as promoiety, it is to cover the undesired functional groups with another functional groups. This prodrug approach is mainly for improving the nasal bioavailability especially for the proteins and peptides to enhance the membrane permeability along with increased enzymatic stability [43]. The prodrug undergoes enzymatic transformation to release the active medicament, when it crosses the enzymatic and membrane barrier.
Particulate drug delivery
Particle design is an increasingly important role in absorption enhancement. Microspheres, nanoparticles and liposomes are all systems which can be used as carriers to encapsulate an active drug. The properties of these can be varied to maximize therapeutic efficacy. Overall, this can result in increased absorption efficacy and stability and reduced toxicity of the active ingredient. Systems can be designed to be mucoadhesive to increase the retention time and facilitate sustained release.
Microspheres are mainly increase the absorption and bioavailability by adhering to the nasal mucosa and increase the nasal residence time of drug [44]. The microspheres prepared by using poly-mers like dextran, chitosan, biodegradable starch microspheres successfully improved the bioavailability of various drugs. Liposomes are amphiphilic in nature are well characterized for favorable permeation of drugs through the biological membranes, so the water soluble drugs have been delivered to nasal drugs. Cationic liposomes are having good permeation capacity than negatively charged anionic liposomes [45].
(A) Microspheres: Microsperes of different materialshave been evaluated in vivo as nasal drug delivery systems. Microspheres of albumin, starch and Diethylamonoethyl (DEAE)-dextran absorb water and form a gel-like layer, which cleares slowly from the nasal cavity.
1) Dextran microspheres were proven bioadhesive microspheres for prolonging the residence time in the nasal cavity. The slowest clearance was detected for DEAE-dextran, where 60% of the delivered dose was still present at the deposition site after 3 hourss[46]. However, these microspheres were not successful in promoting insulin absorption in rats[47]. The insulin was too strongly bound to the DEAE groups to be released by a solution with an ionic strength corresponding to physiological conditions. Structural changes due to the lyophilization process were observed in spheres with insulin incorporated, which probably further decreased the release rate[48].
2) Degradable starch microspheres (DSM) DSM is the most frequently used microsphere system for nasal drug delivery and has been shown to improve the absorption of insulin[49], gentamicin[50], human growth hormone[51], metoclopramide[48] and desmopressin[52]. Insulin administered in DSM to rats resulted in a rapid dose-dependent decrease in blood glucose[53,54]. DSM as a delivery system for insulin (2 IU.kg-1) has also been tested in sheep. The absolute bioavailability was 4.5% and the time to reach maximum effect, i.e., a 50% decrease in plasma glucose, was 60 min[55]. Studies in rabbits have demonstrated that DSM does not induce serious hystopathological changes to the nasal mucosa. Moreover, the DSM was well tolerated by 15 healthy volunteers and did not cause significant changes in mucociliary transport[56]. The effect of starch microspheres on the absorption enhancing efficiency of various enhancer systems with insulin after application in the nasal cavity of the sheep was investigated. The DSM was shown to synergistically increase the effect of the absorption enhancers on the transport of the insulin across the nasal membrane[57].
B) Gels: Chitin and chitosan have been suggested for use as vehicles for the sustained release of drugs. Indomethacin and papaverine hydrochloride were used as model drugs in gel formulations. It was reported that chitin was able to control the release of the above mentioned agents in gel formulation as compared to the powder formulation [58]. Studies showed that cationic polymer chitosan was fairly mucoadhesive in comparison to polycarbophil as a reference substance. They suggested that a strict distinction should be made between mucoadhesive of dry polymers on a wet tissue in air and mucoadhesion of a swollen hydro gel in the presence of a third liquid phase[59]. Nasal absorption of nifedipine from gel preparations, PEG 400, aqueous carbopol gel and carbopol- PEG has been studied in rats. Nasal administration of nifedipine in PEG resulted in rapid absorption and high Cmax; however, the elimination of nifedipine from plasma was very rapid. The plasma concentration of nifedipine after nasal administration in aqueous carbopol gel formulation was very low. The use of PEG 400 in high concentration in humans should be considered carefully because PEG 400 is known to cause nasal irritation in concentrations higher than 10%[60]. The effect of polyacrylic acid gel on the nasal absorption of insulin and calcitonin was investigated in rats. After nasal administration of insulin its absorption from 0.15 w/v polyacrylic acid gel is greater than with 1% w/v gel. There would seem to be an optimum concentration and possibly an optimum viscosity for the polyacrylic acid gel base[61]. The effects of putative bioadhesive polymer gels on slowing nasal mucociliary clearance were investigated using a rat model. The results indicate that all the formulations decreased intranasal mucociliary clearance, thus increasing the residence time of the formulations in the nasal cavity[62].
C) Liposomes: Liposomes have been delivered by various routes. The potential adjuvant effect of liposomes on tetanus toxoid, when delivered via the nasal, oral and i.m. routes compared to delivery in simple solution in relation to the development of a non parenteral immunization procedure, which stimulates a strong systemic immunity. They found that tetanus toxoid entrapped in Distearoylphospatidylcholine (DSPC) liposomes is stable and is taken up intact in the gut[63]. The permeability of liposome entrapping insulin through the nasal mucosa of rabbit has been studied and compared with the permeability of insulin solution with or without pre-treatment by sodium glycocholate (GC). A comparison of the insulin solution and liposome suspension showed that the liposome had permeated more effectively after pre-treatment by GC[64]. The relationship between the rigidity of the liposomal membrane and the absorption of insulin after nasal administration of liposomes modified with an enhancer containing insulin was investigated in rabbits. The nasal administration to rabbits showed high fluidity at 37 °C, caused a high serum glucose reduction, and the reduction effect lasted for 8 hours[65]. The loading and leakage characteristics of the desmopressin containing liposomes and the effect of liposomes on the nasal mucosa permeation and were investigated. The increase of permeability antidiuresis of desmopressin through the nasal mucosa occured in the order positively charged liposomes > negatively charged liposomes > solution[66]. The potential of liposomes as an intranasal dosage formulation for topical application of 5, 6- carboxyfluorescein (CF) was investigated in rats. CF was rapidly absorbed into the systemic circulation and no adhesion of CF to the nasal mucosa was observed. Liposomes suppress drug absorption into the systemic circulation and concurrently increase drug retention in the nasal cavity[67].
C) Gels: Chitin and chitosan have been suggested for use as vehicles for the sustained release of drugs. Indomethacin and papaverine hydrochloride were used as model drugs in gel formulations. It was reported that chitin was able to control the release of the above mentioned agents in gel formulation as compared to the powder formulation [68]. Studies showed that cationic polymer chitosan was fairly mucoadhesive in comparison to polycarbophil as a reference substance. They suggested that a strict distinction should be made between mucoadhesive of dry polymers on a wet tissue in air and mucoadhesion of a swollen hydro gel in the presence of a third liquid phase[69]. Nasal absorption of nifedipine from gel preparations, PEG 400, aqueous carbopol gel and carbopol- PEG has been studied in rats. Nasal administration of nifedipine in PEG resulted in rapid absorption and high Cmax; however, the elimination of nifedipine from plasma was very rapid. The plasma concentration of nifedipine after nasal administration in aqueous carbopol gel formulation was very low. The use of PEG 400 in high concentration in humans should be considered carefully because PEG 400 is known to cause nasal irritation in concentrations higher than 10%[70]. The effect of polyacrylic acid gel on the nasal absorption of insulin and calcitonin was investigated in rats. After nasal administration of insulin its absorption from 0.15 w/v polyacrylic acid gel is greater than with 1% w/v gel. There would seem to be an optimum concentration and possibly an optimum viscosity for the polyacrylic acid gel base[71]. The effects of putative bioadhesive polymer gels on slowing nasal mucociliary clearance were investigated using a rat model. The results indicate that all the formulations decreased intranasal mucociliary clearance, thus increasing the residence time of the formulations in the nasal cavity[72].
D) Cyclodextrins: Several compounds have been investigated for their nasal absorption enhancement potential using cyclodextrins as the optimisers. The most studied types are:-natural cyclodextrin, and hydoxypropyl cyclodextrin. Only cyclodextrin is a compendia substance and is being considered for a GRAS (generally recognised as safe) status [73]. Merkus et al. reported a study which investigated the effects of a dimethyl-cyclodextrin (DM-CD) powder formulation on intranasal insulin absorption in healthy subjects and patients with insulin-dependent diabetes mellitus (IDDM). Mean absolute bioavailabilities of 3.1% and 5.1% were achieved in healthy subjects and diabetics[74], respectively.
E) Fusidic acid derivatives: Sodium tauro-24,25- dihydrofusidate (STDHF) is the most extensively studied among the derivatives of fusidic acid[75]. On the basis of its characteristics, STDHF was considered a good candidate for the transnasal delivery of drugs such as insulin[76], growth hormone[77] and octreotide[78]. Lee et al. determined the radioimmunoactive bioavailability of intranasal salmon calcitonin in 10 healthy human volunteers. The impoved nasal absorption of calcitonin in the presence of STDHF showed a limited transient irritation of the nasal mucosa in some subjects[79]. Hedin et al. studied the intranasal administration of human growth hormone (hGH) in combination with STDHF at 1% concentration in patients with hGH deficiency. They found that in combination with STDHF, the plasma peak of hGH was similar to the endogenous peak[77]. Laursen et al. used a formulation approach in determining the absorption of growth hormone in human subjects using didecanoyl-L-phosphotidylcholine (DDPC) as an enhancer with different concentrations: 0, 4, 8, 16%. They concluded that increasing the relative concentration of DDPC increases the absorption of nasally administered hGH[80]. Drejer et al. studied intranasal administration of insulin with DDPC in healthy human volunteers. They found that intranasal insulin was absorbed in a dose dependent manner with slight or no nasal irritation[81].
F) Phosphatidylcholines(PC): Phosphatidylcholines are surface-active amphihilic compounds produced in biological membranes and liposomes. Several reports have appeared in the literature showing that these phospholipids can be used as enhancers for systemic nasal drug delivery[82]. Newman et al. investigated the distribution of a nasal insulin formulation containing DDPC labeled with 99mTc-human serum albumin (99m Tc-HAS) in human volunteers. From the scintigraphic data, the entire dose from the spray was shown to be deposited in the nasal cavity with no deposition in the lungs[83]. The Novo Nordisk study group reported encouraging results following the nasal administration of an insulin/DDPC microemulsion formulation in human volunteers. The study demonstrated good absorption of insulin whilst pereventing or minimizing nasal irritation[84].
G) Bile salts and surfactants:Commonly used bilesalts are sodium cholate (C), sodium deoxycholate(DC), sodium glycocholate (GC), sodium taurocholate (TC), sodium taurodeoxycholate (TDC), and sodium glycodeoxycholate (GDC). Several studies indicate that bile salts can be good optimisers in nasal drug products, through there are some reports indicating that bile salts cause nasal irritation when used above a concentration of 0.3%[85]. When a solution formulation containing insulin and 1% sodium glycocholate (SGC) dosed nasally significant decreases in serum glucose concentrations were observed and there was a positive correlation between the peak serum insulin levels and the dose of insulin applied[86]. Hirata et al. investigated the efficacy of a nasal insulin formulation containing 1% SGC in healthy volunteers and diabetic patients. The nasal formulation resulted in rapid increases in serum insulin levels and decreases in blood glucose levels in healthy volunteers and diabetics[87]. Comparative studies of the effects of intranasal and subcutaneous insulin on fasting and post-prandial blood insulin and glucose concentrations in non-obese patients with non-insulin-dependent diabetes mellitus (NIDDM) showed significant differences in results. A nasal solution formulation of insulin and 1% SGC, administered as a spray, resulted in a monophasic increase in serum insulin levels[88]. Salzman et al. investigated the efficacy of 1% laureth-9 in enhancing the nasal absorption of insulin in patients with IDDM and non-diabetic controls. Insulin was shown to be rapidly absorbed via the nasal route lowering plasma glucose levels to 50% of basal values after 45 min in normal subjects compared to 50% in 120 min in diabetics[89]. Paquot et al. investigated the metabolic and hormonal consequences of an intranasal insulin formulation administration containing 0.25% laureth-9 in healthy 140 volunteers. Increase in plasma insulin levels from 5 to 38 mU.l-1 at 15 min with decreases in blood glucose concentration from 4.4 to 3.2 mmol. l- 1 at 45 minutes[90]
Permeation enhancers
The permeation enhancers are mainly used for the enhancement of absorption of the active medicament. Generally, the absorption enhancers act via one of the following mechanisms:
* Inhibit enzyme activity;
* Reduce mucus viscosity or elasticity;
* Decrease mucociliary clearance;
* Open tight junctions; and
* Solubilize or stabilize the drug.
Various types of penetration enhancers have been evaluated for organic drugs including surfactants, bile salts, chelators, fatty acid salts, phospholipids, glycyrrhetenic acid derivatives, cyclodextrins and glycols.
Classification of chemical penetration enhancer includes [91]
Surfactants: Polyozyethylene-9-lauryl ether (Laureth-9), Saponin
Bile salts: Trihydroxy salts (glycol and taurocholate), Fusidic acid derivatives (STDHF)
Chelators: Salicylates, Ethylenediaminetetraacetic acid (EDTA)
Fatty acid salts: Oleic acid, Caprylate (C8), Caprate (C10), Laurate (C12)
Phospholipids: Lysophosphatidylcholine (lyso-PC), Di-decanoyl – PC
Glycyrrhetinic acid derivates: Carbenozolone, Glycyrrhizinate
Cyclodextrins: α, ß, and γ- cyclodextrins and their de-rivatives
Glycols: n- glycofurols and n- ethylene glycols
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Structural modifications
Structural Modification of drug without altering pharmacological activity is one of the recent ways to improve the nasal absorption. The chemical modification of drug molecule has been commonly used to modify the physicochemical properties of a drug such as molecular weight, Pka, molecular size and solubility are favorable to improve the nasal absorption of drug. Example, chemical modification of salmon calcitonin to ecatonin (C-N bond replaces the S-S bond) showed better bioavailability than salmon calcitonin [92].
Conclusion
The nasal cavity has a large surface area and a highly vascularized mucosa. Drugs absorbed by the rich network of blood vessels pass directly into the systemic circulation, thereby avoiding first-pass metabolism. Despite the potential of the nasal route, a number of factors limit the intranasal absorption of drug, especially peptide and protein drugs. These are mucus and epithelial barrier, mucociliary clearance and enzymatic activity. Rapid mucociliary clearance of drug formulations that are deposited in the nasal cavity is thought to be an important factor underlying the low bioavailability of drugs administered intranasally. Increasing the residence time of the drug formulation in the nasal cavity, and hence prolonging the period of contact with the nasal mucosa, may improve drug absorption. The physicochemical properties of the drugs is also an important factor that affects the nasal drug absorption; a number of lipophilic drugs have been shown to be completely or almost completely absorbed from the nasal mucosa. The nasal route of administration will probably have great potential for the future development of peptide preparations and other drugs that otherwise should be administered parenterally.
REFERENCES
1) Chien Y.W., Su K.S.E., Chang S.F., Nasal Systemic Drug Delivery, Ch. 1, Marcel-Dekker, New York, 1-77, 1989
2) Mahalaxmi rathananand, D. S. Kumar, A. Shirwaikar, Ravi kumar, D. Sampath kumar, Preparation of Mu-coadhesive Microspheres for Nasal Delivery by Spray Drying, Indian Journal of Pharmaceutical Sciences, 2007,652.
3) Hussain A, Hamadi S, Kagoshima M, Iseki K, Dittert L. Does increasing the lipophilicity of peptides enhance their nasal absorption. J Pharm Sci 1991; 80: 1180-1181. Hussain A.A., Hirai S., Bawarshi R., Nasal absorption of natural contraceptive steroids in rats-progesterone absorption, J. Pharm. Sci.1981, 70, 466–467.
4) Kissel T, Werner U. Nasal delivery of peptides: an in vitro cell culture model for the investigation of transport and metabolism in human nasal epithelium. J Control Rel 1998; 53: 195.203.
5) Ridley D, Perkins AC, Washington N, Wilson CG, Wastie ML, Flynn PO et al. The effect of posture on nasal clearance of bioadhesive starch microspheres. S.T.P. Pharma Sci 1995; 5: 442.6.
6) Illum L. Drug delivery systems for nasal application. In: Hýncal AA, Kas HS, Sumnu M, editors. Third International Pharmaceutical Technology Symposium [Proceedings]. Ankara: Meteksan, 1985.
7) Sarkar MA. Drug metabolism in the nasal mucosa. Pharm Res 1992; 9: 1.9.
8) Brime B, Ballesteros MP, Frutos P. Preparation and in vitro characterization of gelatin microspheres containing Levodopa for nasal administration.
9) Mygind N, Dahl R. Anatomy, physiology and function of the nasal cavities in health and disease. Adv Drug Del Rev 1998; 29: 3.12.)
10) Özer AY. The importance of intranasal route for application of drugs and nasal drug delivery systems. Pharmacia-JTPA 1990; 30: 136.47.
11) Bawarshi RN, Hussain A, Crooks PA. Nasal absorption of 17a- ethinyloestradiol in the rat. J Pharm Pharmacol 1989; 41: 214-215.
12) Hussain A, Hamadi S, Kagoshima M, Iseki K, Dittert L. Does increasing the lipophilicity of peptides enhance their nasal absorption. J Pharm Sci 1991; 80: 1180-1181.
13) Lee V.H.L., Enzymatic barriers to peptide and protein absorption, CRC Crit. Rev. Ther. Drug Carrier Syst. 1988,5, 69–97.
14) Corbo DC, Liu JC, Chien YW. Characterization of the barrier properties of mucosal membranes. J Pharm Sci 1990; 79: 202-206.
15) Ohwaki K, Ando H, Watanabe S, Miyake Y, Effects of dose, pH and osmolarity on nasal absorption of se-cretin in rats, J Pharm Sci 1985;74:550-2
16) Arora P, Sharma S, Garg S. Permeability issues in nasal drug deliv- ery. Drug Discov Today 2002; 7,18, 967-975.
17) Satish BB, adhikrao VY, Amelia MA, Rajkumar M, Bio availability of intranasal drug delivery system, Asian J of Pharmaceutics, 2008; 201-15
18) Gizurarson S, Bechgaard E. Intranasal administration of insulin to humans. Diabetes Res Clin Prac 1991;12:71-84.
19) Schipper NGM, Verhoef JC, Merkus HM. The nasal mucociliary clearance: relevance to nasal drug delivery. Pharm Res 1991; 8: 807.14.
20) Marttin E, Schipper NGM, Verhoef JC, Merkus WHM. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Del Rev 1998; 29: 13.38.
21) Hasani A, Agnew JE, Pavia D, Vora H, Clarke SW. Effect of oral bronchodilators on lung mucociliary clearance during sleep in patients with asthma. Thorax 1993; 48: 287.9.
22) Wolff RK, Dolovich MB, Obminski G, Newhouse MT. Effects of exercise and eucapnic hyperventilation on bronchial clearance in man. J Appl Physiol 1977; 43: 46.50
23) Verra F, Escudier E, Lebargy F, Bernaudin JF, Crèmoux HD, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and non-smokers. Am J Respir Crit Care Med 1995; 151: 630.4.
24) Haxel BR, Schäfer D, Klimek L. Prostaglandin E2 activates the ciliary beat frequency of cultured human nasal mucosa via the second messenger cyclic adenoside monophosphate. Eur Arc Otorhinolaryngol 2001; 258: 230.5.
25) Cho JH, Kwun YS, Jang HS, Kang JM, Won YS, Yoon HR. Longterm use of preservatives on rat nasal respiratorymucosa: effects of benzalkonium chloride and potassium sorbate. Laryngoscope 2000; 110: 312.7.
26) Inagaki M, Sakakura Y, Itoh H, Ukai K, Miyoshi Y. Ma-cromolecu- lar permeability of the tight junction of human nasal mucosa. Rhinology 1985; 23: 213-221.
27) Franz,M.R., Oth,M.P., U.S patent,5232704,1993.
28) Behl, C. R, Pimplaskar, H. K., Sileno, A.P., deMeireles, J. and Romeo, V. D., Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv. Drug Deliv. Rev., 1998, 29, 89-116.
29) Davis, S. S., Nasal vaccines. Adv. Drug Deliv. Rev., 2001, 51, 21-42.
30) A.P., deMeireles, J. and Romeo, V. D., Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv. Drug Deliv. Rev., 1998, 29, 89-116.
31) Ugwoke, M, I., Agu, R, U., Verbeke, N. and Kinget, R., Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives, Adv. Drug Deliv. Rev., 2005, 57, 1640-1665.
32) Talegaonkar, S. and Mishra, P. R., Intranasal delivery: An approach to bypass the blood brain Barrier. Indian J Pharmacol., June 2004, 36(3),140-147.
33) Michael I. Ugwoke, Remigius U. Agu, Norbert Verbeke, Renaat Kinget, Nasal mucoadhesive drug delivery: Background, applications, trends and future perspec-tives, Advanced Drug Delivery Reviews, 2005, 57, 1640 – 1665
34) Sarkar M.A., Drug metabolism in the nasal mucosa, Pharm.Res. 1992, 9, 1–9.
35) Kaliner M., Marom Z ., Patow C., Shelhamer J, Human respiratory mucus, J. Allergy Clin. Immunol. 1984,73, 318 – 323.
36) Bernstein J.M., Reddy M.S.,. Scannapieco F.A, Faden H.S., Ballow M., The microbial ecology and immunol-ogy of the adenoid: implications for otitis media, Ann. N.Y. Acad. Sci.1997,830, 19 – 31.
37) Jadhav, K, R., Gambhire, M, N., Shaikh, I, M., Kadam, V, J. and Pisal, S, S., Nasal Drug Delivery System-Factors Affecting and Applications. Current Drug Therapy, 2007, (2), 27-38.
38) Aurora, J., Development of Nasal Delivery Systems: A Review. Drug Deliv. Technol., 2002, 2(7), 1-8.
39) Liote H, Zahm JM, Pierrot D, Puchelle E. Role of mucus and cilia in nasal mucociliary clearance in healthy subjects. Am. Rev. Respir. Dis. (1989) 140: 132-136.
40) Merkus FWHM, Schipper NGM, Hermens WAJJ, Romeijn SG, Verhoef JC. Absorption enhancers in nasal drug delivery: efficacy and safety. J. Control. Rel. (1993) 24: 201-208.
41) Hussain MA, Koval CA, Shenvi AB, Aungst BJ, Recovery of rat nasal mucosa from the effects of aminopepti-dase inhibitors. J Pharm Sci 1990;79:398-400
42) Donnelly A, Kellaway IW\, Taylor G, Gibson M. Absorp-tion enhancers as tools to determine the route of nasal absorption of peptides.J Drug Target 1998;5:121-7
43) Martin E, Nicolaas GM, Schipper J, Coos V,Frans WH. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Del Rev 1997;29:13-38
44) Edman P, Bjork E, Ryden L. Microspheres as a nasal delivery system for peptidedrugs j controlled release, 1992;21:165-72
45) Chien YW, Chang SF. Intranasal drug delivery for sys-temic medications. Crit Rev Ther Drug Carr Syst 1987;4:67-194.
46) Illum L, Jorgensen H, Bisgaard H, Krogsgaard O, Rossing N. Bioadhesive microspheres as a potential nasal drug delivery system. Int J Pharm 1987; 39: 189.99.
47) Rydèn L, Erdman P. Effects of polymers and microspheres on the nasal absorption of insulin in rats. Int J Pharm 1992; 83: 1.10.
48) Morath LP. Microspheres as nasal drug delivery systems. Adv Drug Del Rev 1998; 29: 185 94.
49) Björk E, Erdman P. Characterization of degradable starch microspheres as a nasal delivery system for drugs. Int J Pharm 1990; 62: 187.92.
50) Illum L, Farraj N, Davis SS. Nasal administration of gentamicin using a novel microsphere delivery system. Int J Pharm 1988; 46: 261.5.
51) Illum L, Farraj NF, Davis SS, Johansen BR, O.Hagan DT. Investigation of the nasal absorption of biosynthetic human growthhormone in sheep . use of a bioadhesive microsphere delivery system. Int J Pharm 1990; 63: 207.11.
52) Critichley H, Davis SS, Farraj NF, Illum L. Nasal absorption of desmopressin in rats and sheep. Effect of a bioadhesive microsphere delivery system. J Pharm Pharmacol 1993; 46: 651.6.
53) Björk E, Erdman P. Degradable starch microspheres as a nasal delivery system for insulin. Int J Pharm 1988; 47: 233.8.
54) Farraj NF, Johansen BR, Davis SS, Illum L. Nasal administration of insulin using bioadhesive microspheres as a delivery system. J Control Rel 1990; 13: 253.61.
55) Hinchcliffe M, Illum L. Intranasal insulin delivery and therapy. Adv Drug Del Rev 1999; 35: 199.234.
56) Illum L, Fisher AN, Jabbal-Gill I, Davis SS. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the nasal absorption of polypeptides. Int J Pharm 2001; 222: 109.19.
57) Vyas SP, Bhatnagar S, Gogoi PJ, Jain NK. Preparation and characterization of HAS propranolol microspheres for nasal administration. Int J Pharm 1991; 69: 5.12.
58) Witschi C, Mrsny R. In vitro evaluation of microparticles and polymer gels for use as nasal platforms for protein delivery. Pharm Res 1999; 16: 382.90.
59) Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm 1992; 78: 43.8.
60) Morimoto K, Tabata H, Morisaka K. Nasal absorption of nifedipine from gel preparations in rats. Chem Pharm Bull 1987; 35: 3041.4.
61) Morimoto K, Morisaka K, Kamada A. Enhancement of nasal absorption of insulin and calcitonin using polyacrylic acid gel. J Pharm Pharmacol 1985; 37: 134.6.
62) Zhou M, Donovan MD. Intranasal mucociliary of putative bioadhesive polymer gels. Int J Pharm 1996; 135: 115.25.
63) Alpar HO, Bowen JC, Brown MRW. Effectiveness of liposomes as adjuvants of orally and nasally administered tetanus toxoid. Int J Pharm 1992; 88: 335.44.
64) Maitani Y, Asano S, Takahashi S, Nakagaki M, Nagai T. Permeability of insulin entrapped in liposome through the nasal mucosa of rabbits. Chem Pharm Bull 1992; 40: 1569.72.
65) Muramatsu K, Maitani Y, Takayama K, Nagai T. The relationship between the rigidity of the liposomal membrane and the absorption of insulin after nasal administration of liposomes modified with an enhancer containing insulin in rabbits. Drug Dev Ind Pharm 1999; 25: 1099. 105.
66) Law SL, Huang K J, Chou HY. Preparation of desmopressin-containing liposomes for intranasal delivery. J Control Rel 2001; 70: 375.82.
67) Iwanaga K, Matsumoto S, Morimoto K, Kakemi M, Yamashita S, Kimura T. Usefulness of liposomes as an intranasal dosage formulation for topical drug application. Biol Pharm Bull 2000; 23: 323.6.
68) Witschi C, Mrsny R. In vitro evaluation of microparticles and polymer gels for use as nasal platforms for protein delivery. Pharm Res 1999; 16: 382.90.
69) Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm 1992; 78: 43.8.
70) Morimoto K, Tabata H, Morisaka K. Nasal absorption of nifedipine from gel preparations in rats. Chem Pharm Bull 1987; 35: 3041.4.
71) Morimoto K, Morisaka K, Kamada A. Enhancement of nasal absorption of insulin and calcitonin using polyacrylic acid gel. J Pharm Pharmacol 1985; 37: 134.6.
72) Zhou M, Donovan MD. Intranasal mucociliary of putative bioadhesive polymer gels. Int J Pharm 1996; 135: 115.25.
73) Behl CR, Pimplaskar HK, Sileno AP, Xia WJ, Gries WJ, Emeireles JC et al. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv Drug Del Rev 1998; 29: 117.33.
74) Merkus FWHM, Verhoef JC, Romeijn SG, Schipper NGM. Absorption enhancing effect of cyclodextrins on intarnasally administered insulin in rats. Pharm Res 1991; 8: 588.92.
75) Deponti R, Lardini E. Use of chemical enhancers for nasal drug delivery. Drug Dev Ind Pharm 1991; 17: 1419.36.
76) Hermens WAJJ, Hooymans PM, Verhoef JC, Merkus FWHM. Effects of absorption enhancers on human nasal tissue ciliary movement in vitro. Pharm Res 1990; 7: 144.6.
77) Hedýn L, Olsson B, Dýczfalusy M, Flyg C, Petersson AS, Rosberg S et al. Intranasal administration of human growth hormone (hGH) in combination with a membrane permeation enhancer in patients with GH deficiency: a pharmacokinetic study. JCE & M 1993; 76: 962.7.
78) Kissel T, Drewe J, Bantle S, Rummelt A, Beglinger C. Tolerability and absorption enhancement of intranasally administered octreotide by sodium taurodihydrofusidate in healthy subjects. Pharm Res 1992; 9: 52.7.
79) Lee WA, Ennis RD, Longenecker JP, Bengtsson P. The bioavailability of intranasal salmon calcitonin in healthy volunteers with and without a permeation enhancer.Pharm Res 1994; 11: 747.50.
80) Laursen T, Ovesen P, Granjean S, Jensen S, Otto J, Jorgensen L et al. Nasal absorption of growth hormone in normal subjects: studies with four different formulations. Ann Pharmacoter 1994; 28: 845.8.
81) Drejer K, Vaag A, Bech K, Hansen P, Sorensen AR, Mygind N. Intranasal administration of insulin with phospholipid as absorption enhancer: pharmacokinetics in normal subjects. Diabet Med 1992; 9: 335.40.
82) Illum L, Farraj NF, Critchley H, Johansen BR ,Davis SS. Enhanced nasal absorption of insulin in rats using lysophosphatidylcholine. Int J Pharm 1989; 57: 49.54.
83) Newman SP, Steed KP, Hardy JG, Wilding IR, Hooper G, Sparrow RA. The distribution of an intranasal formulation of insulin in healthy volunteers: effect of different administration techniques. J Pharm Pharmacol 1994; 46: 657.60.
84) Jorgensen S, Drejer K. Insulin analogues and nasal insulin delivery. In: Bailey CJ, Flatt PR, editors. New antidiabetic drugs. London: Smith-Gordon, 1990; 83.92
85) Moses AC, Gordon GS, Carey MC, Flier JS. Insulin administration intranasally as an insulin-bile salt aerosol. Diabetes 1983; 32: 1040.7.
86) Yokosuka T, Omori Y, Hirata Y, Hirai S. Nasal and sublingual administration of insulin in man. J Jpn Diabetes Soc 1977; 20: 146.52.
87) Hirata Y, Yokosuka T, Kasahara T, Kikuchi M, Ooi K. Nasal administration of insulin in patients with diabetes. In: Baba S, Kaneko T, Yanaihara C, editors. Proceedings of the Symposium on Proinsulin, Insulin and CPeptide, Tokushima, July 1978. Amsterdam: Excerpta Medica, 1979; 319.26.
88) Frauman AG, Jerums G, Louis WJ. Effects of intranasal insulin in non-obese type II diabetics. Diabetes Res Clin Pract 1987; 3: 197.202.
89) Salzman R, Manson JE, Griffing GT. Intranasal aerosolized insulin. Mixed-meal studies and long-term use in type I diabetes. New Engl J Med 1985; 312: 1078.84.
90) Paquot N, Scheen AJ, Franchimont P, Lefebvre P. The intranasal administration of insulin induces significant hypoglycaemia and classical counter-regulatory hormonal responses in normal man. Diabetes Metabol 1988; 14: 31.6.
91) Ramesh RP, Mahesh C, Patil, O. Obire. Nasal Drug deli-very in Pharmaceutical and biotechnology: present and future, e-Journal of Science & Technology, 2009; 3 : 1-21.
92) Hofstee BH. Specificity of esterase. II.Behavior of pan-creatic esterase I and II toward a homologous series of N-fatty acid esters. J Biol Chem 1952; 199:365-71
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









