About Authors:
Saumya Samanta1, Ranabir Chanda2, Jyotishman Bhattacharya3
Benagal School of Technology,
Chuchura, West Bengal, India
*samantasaumya7@gmail.com
ABSTRACT
Formulations of mucoadhesive polymers are of current interest in the design of drug delivery systems. Current uses of mucoadhesive based preparations include ophthalmic solutions, local applications to treat diseases, protein and peptide delivery etc. Several synthetic polymers are used for this purpose. Since the biodegradability of the synthetic polymers are questionable, so polymers and mucoadhesive materials obtained from natural sources are used instead of synthetic polymers. The objective of this article is to review the mechanism of mucoadhesion, techniques that are frequently used to study the adhesion forces. This article also contains structure of G.I tract, types of synthetic and natural polymers, their classifications and method of evaluation of mucoadhesive materials.
REFERENCE ID: PHARMATUTOR-ART-1670
INTRODUCTION:
The term bioadhesion refers to any bond formed between two biological surfaces or a bond between a biological and a synthetic surface. In case of bioadhesive drug delivery, the term bioadhesion is used to describe the adhesion between polymers, either synthetic or natural and soft tissues or the gastrointestinal mucosa. In cases where the bond is formed with the mucus the term mucoadhesion may be used synonymously with bioadhesion. Mucoadhesion can be defined as a state in which two components, of which one is of biological origin, are held together for extended periods of time by the help of interfacial forces. Generally speaking, bioadhesion is a term which broadly includes adhesive interactions with any biological or biologically derived substance, and mucoadhesion is used when the bond is formed with a mucosal surface [1].
OVERVIEW OF MUCOUS MEMBRANE:
Mucous membranes (mucosae) are the moist surfaces, lining the walls of various body cavities such as the gastrointestinal and respiratory tracts. They consist of a connective tissue layer (the lamina propria) above which is an epithelial layer, the surface of which is made moist usually by the presence of a mucus layer. The epithelia may be either single layered (e.g. the stomach, small and large intestine and bronchi) or multilayered/stratified (e.g. in the oesophagus, vagina and cornea). The former contain goblet cells which secrete mucus directly onto the epithelial surfaces, the latter contain, or are adjacent to tissues containing, specialized glands such as salivary glands that secrete mucus onto the epithelial surface. Mucus is present as either a gel layer adherent to the mucosal surface or as a luminal soluble or suspended form. The major components of all mucus gels are mucin glycoproteins, lipids, inorganic salts and water, the latter accounting for more than 95% of its weight, making it a highly hydrated system. The mucin glycoproteins are the most important structure-forming component of the mucus gel, resulting in its characteristic gel-like, cohesive and adhesive properties. The thickness of this mucus layer varies on different mucosal surfaces, from 50 to 450µm in the stomach, to less than 1µm in the oral cavity. The major functions of mucus are that of protection and lubrication (they could be said to act as anti-adherents) [2-6].
Membrane Coating Granules or Cored Granules
In non keratinized epithelia, the accumulation of lipids and cytokeratins in the keratinocytes is less evident and the change in morphology is far less marked than in keratinized epithelia. The mature cells in the outer portion of non-keratinized epithelia become large and flat retain nuclei and other organelles and the cytokeratins do not aggregate to form bundles of filaments as seen in keratinizing epithelia. As cells reach the upper third to quarter of the epithelium, membrane-coating granules become evident at the superficial aspect of the cells and appear to fuse with the plasma membrane, to extrude their contents into the intercellular space. The membrane-coating granules found in non-keratinizing epithelia are spherical in shape, membrane-bounded and measure about 0.2μm in diameter. Such granules have been observed in a variety of other human non keratinized epithelia, including uterine cervix and esophagus [7].
Basement Membrane:
Although the superficial layers of the oral epithelium represent theprimary barrier to the entry of substances from the exterior, it is evident that the basementmembrane also plays a role in limiting the passage of materials across the junction betweenepithelium and connective tissue. A similar mechanism appears to operate in the oppositedirection. The charge on the constituents of the basal lamina may limit the rate of penetrationof lipophilic compounds that can traverse the superficial epithelial barrier relatively easily.
Mucus:
The epithelial cells of buccal mucosa are surrounded by the intercellular ground substance called mucus with the thickness varies from 40μm to 300μm. Though the sublingual glands and minor salivary glands contribute only about 10% of all saliva, together they produce the majority of mucus and are critical in maintaining the mucin layer over the oral mucosa. It serves as an effective delivery vehicle by acting as a lubricant allowing cells to move relative to one another and is believed to play a major role in adhesion of mucoadhesive drug delivery systems, as shown in figure 3. A thorough understanding of the glycoprotein mucin component is very important with regard to understanding the properties of mucus. Mucin glycoproteins may be described as consisting of a basic unit made from a single-chain polypeptide backbone with two distinct regions [8].
Composition of Mucus Layer
Mucus is translucent and viscid secretion which forms a thin, continuous gel blanket adherent to the mucosal epithelial surface. Mucus glycoprotiens are high molecular proteins possessing attached oligosaccharide units containing the composition of mucus [9]
a) L-fucose
b) D-galactose
c) N-acetyl-D-glucosamine
d) N-acetyl-D-galactosamine
e) Sialic acid [2].
ADVANTAGES OF ORAL MUCOADHESIVE DRUG DELIVERY SYSTEMS
1. Prolongs the residence time of the dosage form at the site of absorption, hence
2. increases the bioavailability.
3. Excellent accessibility, rapid onset of action.
4. Rapid absorption because of enormous blood supply and good blood flow rates
5. Drug is protected from degradation in the acidic environment in the GIT.
6. Improved patient compliance
DISADVANTAGES OF MUCOADHESIVE DRUG DELIVERY SYSTEMS:
1. Occurrence of local ulcerous effects due to prolonged contact of the drug possessing ulcerogenic property.
2. One of the major limitations in the development of oral mucosal delivery is the lack of a good model for in vitro screening to identify drugs suitable for such administration.
3. Patient acceptability in terms to taste, irritancy and mouth feel is to be checked [1,10].
MECHANISM OF MUCOADHESION:
As stated, mucoadhesion is the attachment of the drug along with a suitable carrier to the mucous membrane. The mechanism of adhesion of certain macro -molecules to the surface of a mucous tissue is not well understood yet. The mucoadhesive must spread over the substrate to initiate close contact and increase surface contact, promoting the diffusion of its chains within the mucus. Attraction and repulsion forces arise and, for a mucoadhesive to be successful, the attraction forces must dominate. Each step can be facilitated by the nature of the dosage form and how it is administered. For example, a partially hydrated polymer can be adsorbed by the subs-trate because of the attraction by the surface water [11].
Thus, the mechanism of mucoadhesion is generally divided in two steps, the contact stage and the consolida-tion stage (Figure 3).
The first stage is characterized by the contact between the mucoadhesive and the mucous membrane, with spreading and swelling of the formu-lation, initiating its deep contact with the mucus layer [12-13].
In the consolidation step (Figure 3), the mucoadhe -sive materials are activated by the presence of moisture. Moisture plasticizes the system, allowing the mucoadhe-sive molecules to break free and to link up by weak vander Waals and hydrogen bonds [14].
Essentially, there are two theories explaining the consolidation step: the diffusion theory and the dehydration theory. According to diffusion theory, the mucoadhesive molecules and the glycoproteins of the mucus mutually interact by means of interpenetration of their chains and the building of secondary bonds [15].
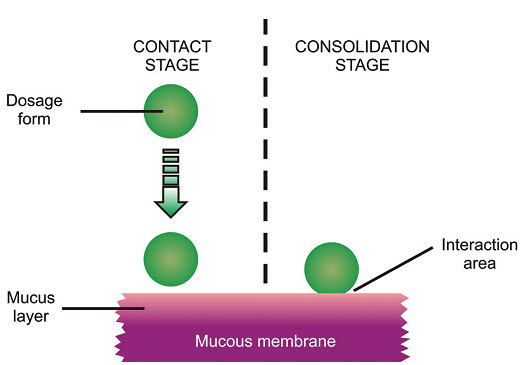
Fig: 3 The two steps of the mucoadhesion process [15]
MUCOADHESION THEORIES:
Although the chemical and physical basis of muco-adhesion are not yet well understood, there are six classical theories adapted from studies on the performance of several materials and polymer-polymer adhesion which explain the phenomenon [12, 14,16].
1. Adsorption theory
According to the adsorption theory, the mucoadhesive device adheres to the mucus by secondary chemical interactions, such as in Van der Waals and hydrogen bonds, electrostatic attraction or hydrophobic interactions. For example, hydrogen bonds are the prevalent interfacial forces in polymers containing carboxyl groups. Such forces have been considered the most important in the adhesive interaction phenomenon because, although they are individually weak, a great number of interactions can result in an intense global adhesion.
In order for diffusion to occur, it is important that the components involved have good mutual solubility, that is, both the bioadhesive and the mucus have similar chemical structures. The greater the structural similarity, the better the mucoadhesive bond [15]
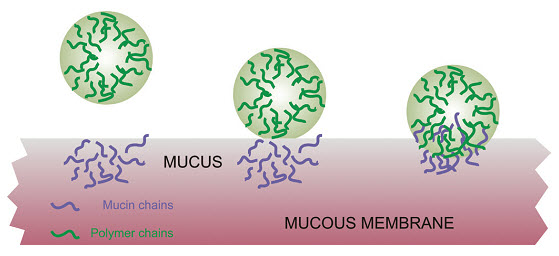
Fig: 4 Secondary interactions resulting from interdiffusion of polymer chains of bioadhesive device and of mucus.
2. Wetting theory
The wetting theory applies to liquid systems which present affinity to the surface in order to spread over it. This affinity can be found by using measuring techniques such as the contact angle. The general rule states that the lower the contact angle then the greater the affinity (Figure 5). The contact angle should be equal or close to zero to provide adequate spreadability.
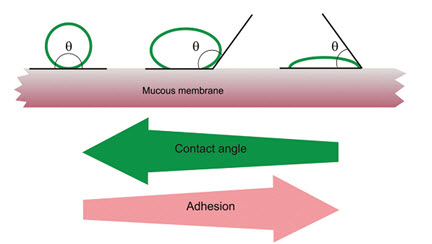
Fig: 5 Schematic diagram showing influence of contact angle between device and mucous membrane on bioadhesion [15]
3. Diffusion theory
Diffusion theory describes the interpenetration of both polymer and mucin chains to a sufficient depth to create a semi-permanent adhesive bond. It is believed that the adhesion force increases with the degree of penetration of the polymer chains. This penetration rate depends on the diffusion coefficient, flexibility and nature of the mucoadhesive chains, mobility and contact time. The adhesion strength for a polymer is reached when the depth of penetration is approximately equivalent to the polymer chain size. In order for diffusion to occur, it is important that the components involved have good mutual solubility, that is, both the bioadhesive and the mucus have similar chemical structures. The greater the structural similarity, the better the mucoadhesive bond.
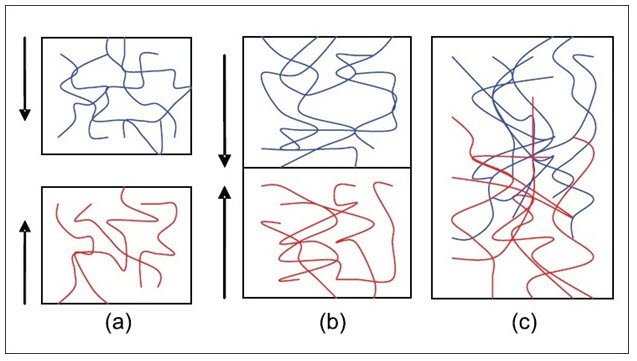
Fig: 6 (a) Schematic representation of the diffusion theory of bioadhesion. Blue polymer layer and red mucus layer before contact; (b) Upon contact; (c) The interface becomes diffuse after contact for a period of time[17]
4. Fracture theory
This is perhaps the most-used theory in studies on the mechanical measurement of mucoadhesion. It analyses the force required to separate two surfaces after adhesion is established (Figure 7). This force, Sm, is frequently calculated in tests of resistance to rupture by the ratio of the maximal detachment force, Fm, and the total surface area, Ao, involved in the adhesive interaction (equation 1):

Since the fracture theory is concerned only with the force required to separate the parts, it does not take into account the interpenetration or diffusion of polymer chains. Consequently, it is appropriate for use in the calculations for rigid or semi-rigid bioadhesive materials, in which the polymer chains do not penetrate into the mucus layer.
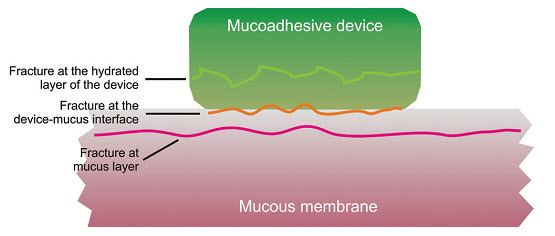
Fig: 7 Regions where the mucoadhesive bond rupture can occur.
5. Mechanical theory
Mechanical theory considers adhesion to be due to the filling of the irregularities on a rough surface by a mucoadhesive liquid. Moreover, such roughness increases the interfacial area available to interactions thereby aiding dissipating energy and can be considered the most important phenomenon of the process.
Lee, Park, Robinson, 2000 had described that it is unlikely that the mucoadhesion process is the same for all cases and therefore it cannot be described by a single theory. In fact, all theories are relevant to identify the important process variables.
The mechanisms governing mucoadhesion are also determined by the intrinsic properties of the formulation and by the environment in which it is applied. Intrinsic factors of the polymer are related to its molecular weight, concentration and chain flexibility. For linear polymers, mucoadhesion increases with molecular weight, but the same relationship does not hold for non-linear polymers. It has been shown that more concentrated mucoadhesive dispersions are retained on the mucous membrane for longer periods, as in the case of systems formed by in situ gelification. After application, such systems spread easily, since they present rheological properties of a liquid, but gelify as they come into contact the absorption site, thus preventing their rapid removal. Chain flexibility is critical to consolidate the interpenetration between formulation and mucus.
Environment-related factors include pH, initial contact time, swelling and physiological variations. The pH can influence the formation of ionizable groups in polymers as well as the formation of charges on the mucus surface. Contact time between mucoadhesive and mucus layer determines the extent of chain interpenetration. Super-hydration of the system can lead to build up of mucilage without adhesion. The thickness of the mucus layer can vary from 50 to 450 µm in the stomach to less than 1µm in the oral cavity. Other physiological variations can also occur with diseases [18].
Determination of Mucoadhesivenss of Polymers
1. Shear Stress Measurement
The shear stress measure the force that cause a mucoadhesive to slide with respect to the mucus layer in directional parallel to their place of contact of adhesion. Different concentration of mucoadhesive agent solution like 1%, 2%, 3% w/v using the test polymer was prepared. A specified amount of prepared solution was spread on 3 glass plate. Another clean slide was placed over the first plate and made to spread the polymer solution uniformly in between two glass plates by placing 100g weight on the glass plates. It was allowed undistributed for 15, 30 and 60 min then one side of glass plate was fixed in a hook and the other was collected to a twin passing over a pulley and at the end of pan was attached. After the said times 15, 30 and 60 min weight was placed in an increasing manner till the plates attached with polymer got detached. The weight which just detaches, were noted and the average values were recorded.19-20
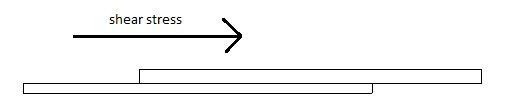
Fig: 8 Schematic of the Shear Stress Measurement method.
2. Falling sphere method
To characterize the mucoadhesive strength, the falling sphere method was used for that a clean burette was taken and filled with fresh goat intestinal mucus solution and fixed in a stainless steel tube. Mustard grains were taken and dipped in polymer solutions of various concentrations (1.0, 2.0 and 3.0% w/v) and then each grain were slowly placed at the top of the mucus layer. Time taken by the grain to fall 50 divisions in the burette was noted and values were tabulated.[21-22]
[Preperation of Goat Intestinal Mucus Solution: Crude mucus was obtained by scraping goat intestine and was collected and diluted with twice of its volume with distilled water.The mixture was entrifuged for 30 min at 1200 r.p.m. The supernatant and sedimented portions were discarded, and the middle layer was collected for further use. The mucus was stored below -200 C until used. The pH of the collected mucuswas 5.5.]
3. Wilhelmy plate method
In this method small glass plates were coated uniform by synthetic polymer and natural bioadhesive material solution to be tested and dried at 600C. The prepared coated plates were immersed in goat intestinal mucus solution (pH=5.5), or USP simulated intestinal fluid (pH=6.0), or USP simulated gastric fluid (pH=1.2), for 5, 10, 15, and 30 min, at room temperature. The force required to pull the plate out of the solution was determined under constant experimental conditions.23
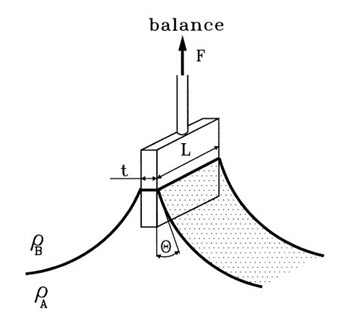
Fig: 9 A schematic of the Wilhelmy plate method.
4. Park and Robinson Method
In this method, the force required to separate bio-adhesive sample from freshly excised rabbit stomach was determined using a modified tensiometer. A section of the tissue, having the mucus side exposed, was secured on a weighted glass vial placed in a beaker containing U.S.P. simulated intestinal fluid. Another section of the same tissue was placed over a rubber stopper, again with the mucus side exposed, and secured with a vial cap. Then a small quantity of synthetic polymer or natural mucoadhesive agent was placed between the two mucosal tissues. The force used to detach the polymers or nature mucoadhesive agents from the tissue was then recorded. Experimentations were performed at room temperature.24
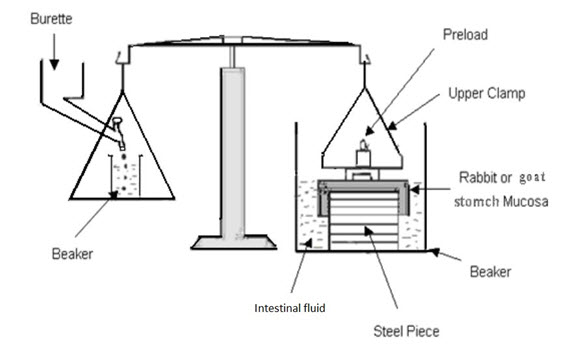
FIG-10: A schematic of the Park and Robinson Method
Factors affecting Mucoadhesion
1. Polymer related factors:
i) Molecular weight
ii) Concentration of active polymer
iii) Flexibility of polymer chains
iv)Special confirmation
v) Swelling
2. Environment related factors:
i)pH of polymer - substrate interface
ii) Applied strength
iii) Initial contact time
3. Physiological factors:
i) Mucin turns over
ii) Disease state
1. Polymer-Related Factors
Molecular weight: The optimum molecular weight for maximum bioadhesion depends upon type of mucoadhesive polymer atissue. It is generally understood that the threshold required for successful bioadhesion is at least 100000molecular weight. For example, polyethylene glycol (PEG), with a molecular weight of 20000, has littleadhesive character, whereas PEG with 200000 molecular weight has improved, and PEG with 400000 hassuperior adhesive properties. The fact that mucoadhesiveness improves with increasing molecular weight forlinear polymers implies two things: (1) interpenetration is more critical for a low-molecular-weight polymer tobe a good mucoadhesive, and (2) entanglement is important for high-molecular-weight polymers. Adhesivenessof a nonlinear structure, by comparison, follows a quite different trend. The adhesive strength of dextran, with ahigh molecular weight of 19500000 is similar to that of PEG, with a molecular weight of 200000. The reasonfor this similarity may be that the helical conformation of dextran may shield many of the adhesive groups,which are primarily responsible for adhesion, unlike the conformation of PEG.
Concentration of active polymer: There is an optimum concentration for a mucoadhesive polymer to produce maximum bioadhesion. In highlyconcentrated system, beyond the optimum level, however, the adhesive strength drops significantly because thecoiled molecules become separated from the medium so that the chain available for interpenetration becomeslimited.
Flexibility of polymer chains: Chain flexibility is critical for interpenetration and entanglement. As water soluble polymers become crosslinked,the mobility of an individual polymer chain decreases and thus the effective length of the chain that canpenetrate into the mucus layer decreases, which reduces mucoadhesive strength.
Spatial conformation: Besides molecular weight or chain length, spatial conformation of a molecule is also important. Despite a highmolecular weight of 19500000 for dextrans, they have adhesive strength similar to that of PEG, with amolecular weight of 200000. The helical conformation of dextran may shield many adhesively active groups,primarily responsible for adhesion, unlike PEG polymers, which have a linear conformation.
Swelling: Swelling characteristics are related to the mucoadhesive itself and its environment. Swelling depends on thepolymer concentration, the ionic strength, and the presence of water. During the dynamic process ofbioadhesion, maximum bioadhesion in vitro occurs with optimum water content. Overhydration results in theformation of a wet slippery mucilage without adhesion.
2.Environment-Related Factors
pH of polymer–substrate interface: pH can influence the formal charge on the surface of the mucus as well as certain ionizable mucoadhesivepolymers. Mucus will have a different charge density depending on pH due to the difference in dissociation offunctional groups on the carbohydrate moiety and the amino acids of the polypeptide backbone. Some studieshad shown that the pH of the medium is important for the degree of hydration of cross-linked polycyclic acid,showing consistently increased hydration from pH 4 through pH 7, and then a decrease as alkalinity or ionicstrength increases, for example polycarbophil does not show a strong mucoadhesive property above pH 5because uncharged, rather than ionized, carboxyl group reacts with mucin molecule, presumably throughnumerous hydrogen bonds. However, at higher pH, the chain is fully extended due to electrostatic repulsion ofthe carboxyl ate anions.
Applied strength: To place a solid mucoadhesive system, it is necessary to apply a defined strength. Whatever the polymer, poly(acrylic acid/di-vinyl benzene) or carbopol 934, the adhesion strength increases with the applied strength or withthe duration of its application, up to an optimum. The pressure initially applied to the mucoadhesive tissuecontact site can affect the depth of interpenetration. If high pressure is applied for a sufficiently long period oftime, polymers become mucoadhesive even though they do not have attractive interactions with mucin.
Initial contact time: Contact time between the mucoadhesive and mucus layer determines the extent .of swelling and interpenetrationof the mucoadhesive polymer chains. More mucoadhesive strength increases as the initial contact timeincreases.
3. Physiological Factors
Mucin turnover: The natural turnover of mucin molecules from the mucus layer is important for at least two reasons. Firstly, themucin turnover is expected to limit the residence time of the mucoadhesives on the mucus layer. No matter howhigh the mucoadhesive strength, they are detached from the surface due to mucin turnover. The turnover ratemay be different in the presence of mucoadhesives, but no information is available on this aspect. Secondly,mucin turnover results in substantial amounts of soluble mucin molecules. These molecules interact withmucoadhesives before they have chance to interact with the mucus layer. Surface fouling is unfavorable formucoadhesion to the tissue surface. Mucin turnover may depend on the other factors such as the presence offood. The gastric mucosa accumulates secreted mucin on the luminal surface of the tissue during the early stagesof fasting. The accumulated mucin is subsequently released by freshly secreted acid or simply by the passage ofingested food; the exact turnover rate of the mucus layer remains to be determined. Lehr et al. calculated amucin turnover time of 47–270 min. The ciliated cells in the nasal cavity are known to transport the mucus tothe throat at the rate of 5 mm/min. The mucociliary clearance in the tracheal region has been found to be at therate of 4–10 mm/min.
Disease state: The physiochemical properties of the mucus are known to change during disease conditions such as the commoncold, gastric ulcers, ulcerative colitis, cystic fibrosis, bacterial, and fungal infections of female reproductivetract, and inflammatory conditions of the eye. The exact structural changes taking place in mucus under theseconditions are not clearly understood. If mucoadhesives are to be used in the disease states, the mucoadhesiveproperty needs to be evaluated under the same conditions [24, 25]
Mucoadhesive Polymers
Mucoadhesive polymers are water-soluble and water insoluble polymers, which are swellable networks, jointed by cross-linking agents. These polymers possess optimal polarity to make sure that they permit sufficient wetting by the mucus and optimal fluidity that permits the mutual adsorption and interpenetration of polymer and mucus to take place. Mucoadhesive polymers that adhere to the mucin-epithelial surface can be conveniently divided into three broad classes:
• Polymers that become sticky when placed in water and owe their mucoadhesion to stickiness.
• Polymers that adhere through nonspecific, non-covalent interactions that is primarily electrostatic in nature (although hydrogen and hydrophobic bonding may be significant).
• Polymers that bind to specific receptor site on tile self surface.
Characteristics of an ideal mucoadhesive polymer
An ideal mucoadhesive polymer has the following characteristics[29-30]:
• The polymer and its degradation products should be nontoxic and should be non-absorbable from the gastrointestinal tract.
• It should be nonirritant to the mucous membrane.
• It should preferably form a strong non-covalent bond with the mucin-epithelial cell surfaces.
• It should adhere quickly to most tissue and should possess some site-specificity.
• It should allow daily incorporation to the drug and offer no hindrance to its release.
• The polymer must not decompose on storage or during the shelf life of the dosage form.
• The cost of polymer should not be high so that the prepared dosage form remains competitive. [26, 27]
Molecular characteristics
The properties exhibited by a good mucoadhesive may be summarized as follows:
• Strong hydrogen bonding groups (-OH, -COOH).
• Strong anionic charges.
• Sufficient flexibility to penetrate the mucus network or tissue crevices.
• Surface tension characteristics suitable for wetting mucus/mucosal tissue surface.
• High molecular weight.
Although an anionic nature is preferable for a good mucoadhesive, a range of nonionic molecules (e.g., cellulose derivatives) and some cationic (e.g., Chitosan) can be successfully used [28, 29].
A short list of mucoadhesive polymers is given below:
Synthetic polymers:
Cellulose derivatives (methylcellulose, ethyl cellulose, hydroxy-ethylcellulose, Hydroxyl propyl cellulose,etc., Poly (hydroxyethyl methylacrylate), Poly (ethylene oxide), Poly (vinyl pyrrolidone), Poly (vinyl alcohol).
Natural polymers:
Tragacanth, Sodium alginate, Karaya gum, Guar gum, Xanthan gum, Lectin, Soluble starch, Gelatin, Pectin, Chitosan, sodium alginate.
A list of synthetic and natural polymers is given in Table 1.
Table 1: List of Synthetic and Natural Polymers
|
Criteria |
Catagories |
Examples |
|
Source |
Semi Natural |
Agarose, Chitosan, Gelatin, Hyaluronic Acid, Various Gums (guar, xanthan, gellan, carragenan, pectin and sodium alginate). |
|
Cellulose Derivatives (CMC, thiolated CMC, Sodium CMC, HEC, HPMC, MC, MHEC) |
Thiloated CMC, HEC, HPC, Poly (acrylic acid)-based polymers (CP, PC, PAA), Polyacrylates, Poly(methylvinylether-co-methacrylic acid), PVA. |
|
|
Aqueous Solubility |
Water Soluble |
CP, HEC, HPC (waterb 38 8C), HPMC (cold water), PAA, Sodium CMC, Sodium Alginate. |
|
Water Insoluble |
Chitosan (soluble in dilute aqueous acids), EC, PC. |
|
|
Charge |
Cationic |
Aminodaxtran, Chitosan, (DEAE)-dextran, TMC |
|
Anionic |
Chitosan-EDTA,CP,CMC,Pectin,PAA,PC, Sodium Alginate, Sodium CMC, Xanthan Gum. |
|
|
Non-ionic |
Hydroxyethyl starch, HPC, Poly (Ethylene Oxide), PVA, PVP, Scleroglucan. |
Disadvantages of synthetic polymers in pharmaceutical sciences
- The synthetic polymers have certain disadvantages such as high cost, toxicity, environmental pollution during synthesis, non-renewable sources, side effects, and poor patient compliance.
- Acute and chronic adverse effects (skin and eye irritation) have been observed in workers handling the related substances methyl methacrylate and poly-(methyl methacrylate) (PMMA).[30] Reports of adverse reactions to povidone primarily concern the formation of subcutaneous granulomas at the injection site produced by povidone. There is also evidence that povidone may accumulate in organs following intramuscular injections.[31]
- Acute oral toxicity studies in animals have indicated that carbomer-934P has a low oral toxicity at a dose of up to 8 g/kg. Carbomer dust is irritating to the eyes, mucous membranes and respiratory tract. So, gloves, eye protection and dust respirator are recommended during handling [32].
Studies in rats have shown that 5% polyvinyl alcohol aqueous solution injected subcutaneously can cause anemia and can infiltrate various organs and tissues [33]. Some disadvantages of biodegradable polymers used in tissue engineering applications are their poor biocom-patibility, release of acidic degradation products, poor processing ability and rapid loss of mechanical properties during degradation. It has been shown that poly glyco-lides, polylactides and their co-polymers have an acceptable biocompatibility but exhibit systemic or local reactions due to acidic degradation products. An initial mild inflammatory response has been reported when using poly-(propylene fumarate) in rat implant studies [34]
Advantage of natural polymer:
The various advantages of natural plant based materials include:
1. Biodegradable: Naturally available biodegradable polymers are produced by all living organisms. They represent truly renewable source and they have no adverse impact on humans or environmental health (e.g., skin and eye irritation).
2. Biocompatible and non-toxic: Chemically, nearly all of these plant materials are carbohydrates composed of repeating sugar (monosaccharides) units. Hence, they are non- toxic.
3. Low cost: cheaper to use as natural sources. the production cost is less compared with the synthetic material. In India and many other developing countries are dependent on agriculture and they are large amount of money investment on agricultures.
4. Environmental-friendly processing: There are many types of natural compounds obtained from different plant sources which are widely used in pharmaceutical industry and collected in large quantities due to the simple production processes involved.
5. Local availability (especially in developing countries): In India and similar developing countries, there is promotion for the production of plants as pharmaceutical excipients being done by government and it also provide the facilities for bulk production, like gum and mucilages because of there wide applications in industries.
6. They have better patient tolerance as well as public acceptance: There is less chance of side and adverse effects with natural materials compared with synthetic one. For example, povidone, PMMA.
Characteristics of some natural mucoadhesive polymers are described below:-
Chitosan
Chitosan a derivative form of chitin is a naturally occurring biopolymer. Chitosan is a linear polysaccharide composed of randomly distributed β- (1-4)-linked D-glucosamine (deacetylated unit) and Nacetyl- D-glucosamine (acetylated unit). Commercial chitosan is derived from the shells of shrimp and other sea crustaceans, including Pandalus borealis.
Properties of chitosan
- Used in trans-dermal drug delivery.
- Mucoadhesive nature
- Chitosan ability to produce many different form when.
- In drug delivery, it shows positive charge under acidic conditions.
- Chitosan is insoluble in neutral and basic environments.
- Chitosan may form many translational metal ions.
- Ability to attach itself to other molecules.
- Ability of specific cellular action for target drugs.
- It has bacteriostatic and fungistatic effect [35].
Advantage of chitosan
Chitosan have good biocompatibility and low toxicity that makes it a good pharmaceutical excipient in both conventional and novel applications [36].
Application of chitosan
Various applications of chitosan and its derivatives in pharmaceutical field:
- It is a good diluent for direct compression of tablets formulation.
- It is used as binder for wet granulation.
- Chitosan shows controlled release of drugs from tablets, granules and in film.
- It increases viscosity in solutions during hydrogels preparation.
- Chitosan improves the dissolution of poorly soluble drugs and enhance the absorption of drug in nasal and oral drug delivery system.
- A novel mucoadhesive polymer used for transmucosal drug delivery system.
- Microcrystalline chitosan has high capacity for retaining water so this is advantageous in development of slow release formulation, formulation of gels that control drug release.
- The hydrophilic nature of microcrystalline chitosan aid in, controlling rate of drug release for mucoadhesive formulations in stomach.
- The cationic form of chitosan polymer has potential for DNA complexation and could be useful for non viral vectors for gene therapy. Chitosan protects DNA against DNAase degradation [37-43].
Guar gum
Guar gum is naturally occurring form of galactomannan and also called guaran20. It is primarily ground endosperm of guar beans. Guar gum contains about 80% galactomannan, 12% water, 5% protein, 2% acid soluble ash, and 0.7% fat. The molecular weight of guar gum is approximately 1 million that give high viscosity in solution. The high viscosity of guar gum is due to its long chain structure and high molecular weight. Guar gum is a polysaccharide composed of the sugars galactose and mannose [44-46].
Properties of guar gum
Guar gum is rapidly soluble in cold and hot water but insoluble in many organic solvents. Guar gum has excellent properties such as emulsifying agent, thickening, stabilizing and film forming agent. Guar gum has ability to control rheology by water phase management. The viscosity of guar gum is affected by temperature, pH, salts and other solids [47]. Guar gum is used in colon delivery due to its drug release retarding property. Guar gum has also susceptable to microbial degradation in the large intestine.
Pharmaceutical Application of Guar gum
In the pharmaceutical industry guar gum is used as binder or as disintegrates in tablets. It is also used in some bulk-forming laxatives. In cosmetics and toiletries industries, guar gum is applicable as thickener in toothpastes and conditioner in shampoos.
Advantage of of Guar gum
It is non-toxic, biocompatible and can be used without any side effect [45].
Tragacanth
Tragacanth is a naturally occurring dried gum obtained from Astragalus gummifer and other species of Astragalus grown in Western Asia.
Properties of Tragacanth
- Practically insoluble in water, ethanol (95%), and other organic solvents. Although insoluble in water, tragacanth gum swells rapidly in 10 times its own weight of either hot or cold water to produce viscous colloidal solutions or semigels.
- The gum consists of a mixture of water-insoluble and water-soluble polysaccharides. Bassorin, which constitutes 60–70% of the gum, is the main water-insoluble portion, while the remainder of the gum consists of the water-soluble material tragacanthin. Tragacanth gum has an approximate molecular weight of 840 000.
- It is slightly acidic in nature.
- Tragacanth gum is a viscous, odorless, tasteless and water-soluble mixture of polysaccharides.
Pharmaceutical application of Tragacanth
- It is used as binder and diluent in tablet formulations.
- Tragacanth used as an emulsifying and suspending agent in a variety of pharmaceutical formulations. It is used in creams, gels, and emulsions at various concentrations accord-ing to the application of the formulation and the grade of gum used.
- Used as thickening agent.
- Tragacanth gum is also used similarly in cosmetics and food products [48].
Advantageof Tragacanth
Tragacanth has been used for many years in oral oral pharmaceu-tical formulations and food products, and is generally regarded as an essentially nontoxic material. Tragacanth has been shown to be noncarcinogenic [49].
Sodium alginate
Alginic acid or alginate is an anionic polysaccharide, also called as algin and obtained in the cell walls of brown algae. It has ability of binding with water and forming a viscous gum. Alginic acid is capable of absorbing 200-300 times its own weight in water when water extracted from alginate [50]. Alginate is mainly extracted from seaweed. Alginic acid is mainly produced by two bacterial genera such as Pseudomonas and Azotobacter. These play an important role in the preparation of its biosynthesis pathway [51]. Sodium alginate is the sodium salt of alginic acid, which is a mixture of polyuronic acids composed of residues of D-mannuronic acid and L -guluronic acid.52
Properties of Sodium alginate
- pH 7.2 for a 1% w/v aqueous solution.
- Practically insoluble in ethanol (95%), ether, chloro-form, and ethanol/water mixtures in which the ethanol content is greater than 30%. Also, practically insoluble in other organic solvents and aqueous acidic solutions in which the pH is less than 3. Slowly soluble in water, forming a viscous colloidal solution.
- Typically, a 1% w/v aqueous solution, at 208 C, will have a viscosity of 20–400 cP. Viscosity may vary depending upon concentration, pH, temperature, or the presence of metal ions. Above pH 10, viscosity decreases.[53]
Pharmaceutical application of sodium alginate
Sodium alginate is used in a variety of oral and topical pharmaceutical formulations.
- In tablet formulations, sodium alginate may be used as both a binder and disintegrant;54
- It is used as emulsifier.
- Sodium alginate has also been used in the preparation of sustained-release oral formulations since it can delay the dissolution of a drug from tablets,[55-57] capsules,[58] and aqueous suspensions.[59]
- In topical formulations, sodium alginate is widely used as a thickening and suspending agent
- Recently, sodium alginate has been used for the aqueous microencapsulation of drugs,[60]
- It has also been used in the formation of nanoparticles.[61]
- It is used for pulling radioactive toxins from the body because of their good chelating property.
- It is also used in immobilizing enzymes by inclusion.[62-63]
- The adhesiveness of hydrogels prepared from sodium alginate has been investigated[64] and drug release from oral mucosal adhesive tablets,[65] and buccal gels,[66-67] based on sodium alginate have been reported.
Advantage ofsodium alginate
Sodium alginate is widely used in cosmetics, food products, and pharmaceutical formulations, such as tablets and topical products, including wound dressings. It is generally regarded as a nontoxic and nonirritant material.
Tamarind Gum
Tamarind xyloglucan is obtained from the endosperm of the seed of the tamarind tree. Tamarind Gum, also known as Tamarind Kernel Powder (TKP) is extracted from the seeds. It is polysaccharide in nature.
Physical properties of Tamarind Gum
Tamarind kernel powder disperses and hydrates quickly in cold water but does not reach maximum viscosity unless it is heated for 20-30 mins. The solution exhibits typical non newtonian flow properties common to most other hydrocolloids.[68] The functional properties of tamarind kernel powder of protein concentrates were reported.[69] The rheological properties of tamarind kernel powder suspension showed that suspension behaved like nonnewtonian, pseudoplastic fluid with yield stresses and exhibited thixotropic characteristics. An increasing concentration produces increase in nonnewtonian behavior as in consistency latex, yields stress and apparent viscosity.[70]
Application of Tamarind Gum
1. It has been used as excipient in hydrophilic drug delivery system.
2. As thichkening agent
3. As binder in tablet dosage form
4. As stabilizer in emulsion
5. In Ophthalmic drug delivery
Tamarind seed polysaccharide is used for production of thickened ophthalmic solutions having a pseudoplastic rheological behavior and mucoadhesive properties. Solution is used as a vehicle for sustained release ophthalmic drugs.[71]
6. In sustained drug delivery, it is used as potential polysaccharide having high drug holding capacity for sustained release of verapamil hydrochloride. The release pattern was found to be comparable with matrices of other polysaccharide polymers such as ethyl cellulose, hydroxyethyl cellulose and hydroxypropylmethyl cellulose, as well as the commercially available sustained release tablets (isoptin SR).[72]
Advantage of Tamarind Gum
Tamarind gum shows non carcinogenicity, biocompatibility, mucoadhesivity, high drug holding capacity and high thermal stability . This has led to its application as excipient in hydrophilic drug delivery system. In tablet compression the hardness and friability were better but the disintegration time was more in case of tamarind gum prepared from raw seeds. This proved the excellent binding efficiency of the tamarind gum prepared in either methods comparing acacia and tragacanth.[73]
Ispaghula husk (Psyllium)
Psyllium seed husks, also known as ispaghula, isabgol, or simply as psyllium, are portions of the seeds of the plant Plantago ovata.
Properties of Ispaghula husk (Psyllium)
It is mucilaginous, laxative, demulcent, emollient, some microstatic effects on intestinal microorganisms are also attributed to the seeds and husk. it is mucilaginous, laxative, cooling, demulcent, emollient, some microstatic effects on intestinal microorganisms are also attributed to the seeds and husk. The mucilage of isabgol consists of pentosan and aldobionic acid, which on hydrolysis yield arabinose, galactose, galactouronic acid and rhamnose.[74] The Isabgol mucilage has the property to swell upto 10-14 times of its original volume.
Application of Ispaghula husk (Psyllium)
Psyllium seed husk is used as binder, disintegrant and release retardant/sustained release agent.
It has been reported that, novel mucoadhesive beads were developed and evaluated for oral controlled release of the hypoglycaemic agent gliclazide with the use of ispaghula husk.[75] In the novel mucoadhesive drug delivery system, ispaghula has been used in preparation of alginate-ispaghula beads containing itraconazole(antifungal agent) were prepared by employing the ionotropic gelation method using calcium chloride (CaCl2) as a counter ion.[76] It has been used in the development a novel hydrogel system for the controlled drug delivery device,(Baljeet Singh et al.)
In an attempt, psyllium and acrylic acid based pH sensitive novel hydrogels for the use in colon specific drug delivery was studied. The hydrogel was evaluated for the swelling mechanism and drug release mechanism from the polymeric networks. The effects of pH on the swelling kinetics and release pattern of drugs have been studied by varying the pH of the release medium. It has been observed that swelling and release of drugs from the hydrogels occurred through non-Fickian or anomalous diffusion mechanism in distilled water and pH 7.4 buffer. It shows that the rate of polymer chain relaxation and the rate of drug diffusion from these hydrogels are comparable.
Advantage of Ispaghula husk (Psyllium)
It is non-toxic, biocompatible and can be used without any side effect.
Khaya gum
Khaya gum is a polysaccharide obtained from the incised trunk of the tree Khaya grandifoliola (family Meliaceae).
Properties of Khaya gum
1. It is known to contain highly branched polysaccharides consisting of D galactose, L-rhamnose, D-galacturonic acid and 4-O-60 methyl-D-glucoronic acid .
2. Powered gum is colourless to to reddish brown.
3. pH of the mucilage at 28˚C is 4.2
4. It swells upto 10 times of its original volume in water
5. It is soluble to some extent in hot and cold water but insoluble in ethanol, chloroform and other organic solvents.[77]
Application of Khaya gum
1. Khaya gum has been shown to be useful as a binding agent in tablet formulations. Khaya gum is a hydrophilic polymer and has been shown to possess emulsifying properties comparable with acacia gum.The fact that Further work has also shown its potential as a directly compressible matrix system in the formulation of mucoadhesive controlled release tablets.[78]
2. As suspending agent
3. As thickening agent
Advantage of Khaya gum
1. the gum is naturally available, inexpensive and non-toxic has also fostered the interest in developing the gum for pharmaceutical use.
2. Khaya gum has been successfully evaluated as a controlled release agent in comparison with hydroxypropylmethylcellulose (HPMC) using paracetamol (water soluble) and indomethacin (water insoluble) as model drugs. Khaya gum matrices provided a controlled release of paracetamol for up to 5 h. The release of paracetamol from khaya gum matrices followed time independent kinetics and release rates were dependent on the concentration of the drug present in the matrix. A combinationof khaya gum and HPMC gave zero-order time-independent release kinetics.[79]
Fenugreek seeds
Trigonella Foenum-graceum, commonly known as Fenugreek, is an natural harb of the leguminosea family.[80]
Properties of Fenugreek seeds
Fenugreek seeds contain a high percentage of mucilage (a natural gummy substance present in the coatings of many seeds). Although it does not dissolve in water, mucilage forms a viscous tacky mass when exposed to fluids. Like other mucilage- containing substances, fenugreek seeds swell up 82 and become slick when they are exposed to fluids
Application of Fenugreek seeds
1. As release retardant
2. As binding/granulating agent in tablet formulation
3. Mucoadhesive in nature
Advantage of Fenugreek seeds
Fenugreek gum thicken ingested food to form a gel in stomach trapping fat, sugars and starch hydrolyzing, amylase enzymes to slow down sugar absorption. Thus, it is good for obese and diabetic persons. The gel, which appears like 'fat' inside the body, signals the gall bladder to empty bile into the stomach. The gel then irreversibly traps lipid-emulsifying bile salts and prevents their reabsorption. Thus, emulsification and absorption of lipids including cholesterol results in lowering of blood lipid. This in turn reduces hypertension and chance of heart attack.
The advantage of fenugreek husk over starch as a binding agent was that it could be used as a cold binder whereas starch has to be heated. [81]
References
1. Pranshu Tangri, N.V. Satheesh Madhav, mucoadhesive drug delivery: mechanism and methods of evaluation / International Journal of Biopharmaceutics. 2011; 2 1 : 36-46.
2. Vinod KR, Rohit Reddy T, Sandhya S, David Banji, Venkatram Reddy. Critical Review on Mucoadhesive Drug Delivery Systems. Hygeia Journal of Drugs and Medicines vol.4 (1), April2012 –September2012: 7-28
3. Marriott C, Gregory NP. Mucus physiology and pathology. In: Lanaerts V, Gurny R, editors, Bioadhesive Drug Delivery Systems, Florida:CRC Press; 1990,1–24
4. Allen A, Cunliffe WJ, Pearson JP, Venables CW, The Adherant Gastric Mucus Gel Barrier in Man and Changes in Peptic Ulceration. J. Intern. Med.228, 1990,83–90.
5. Kerss S, Allen A, Garner A. A Simple Method for Measuring the Thickness of the Mucus Gel Layer Adherent to Rat, Frog and Human Gastric Mucosa: Influence Of Feeding, Prostaglandin, Nacetylcysteine and Other Agents, Clin. Sci.63, 1982,187–95.
6. Sonju T, Cristensen T.B, Kornstad L, Rolla G. Electron Microscopy, Carbohydrate Analysis And Biological Activities Of The Proteins Adsorbed In Two Hours To Tooth Surfaces Invivo, Caries Res.8, 1974,113– 22.
7. Yajaman Sudhakar, Ketousetuo Kuotsu, A.K. Bandyopadhyay. Buccal bioadhesive drug delivery: A promising option for orally less efficient drugs. Journal of Control Release 2006; 114: 15-40.
8. Fiebrig I. Harding SE, Rowe, AJ, Hyman, SC, Davis. Transmission electron microscopy studies on pig gastric mucin and its interactions with chitosan. Carbohydr. Polym 1995; 28: 239-244.
9. Lehr CM. Lectin-Mediated Drug Delivery: The Second Generation of Bioadhesives. J Control Release. 2000 Mar 1;65(1-2):19-29.
10. Punitha S, Girish Y. Polymers in mucoadhesive buccal drug delivery system – A review. Int. J. Res. Pharm. Sci. Vol-1, Issue-2, 170-186, 2010
11. Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci., v.89, n.7, p.850-866, 2000.
12. Hagerstrom H. Polymer gels as pharmaceutical dosage forms: rheological performance and physicochemical interactions at the gel-mucus interface for formulations intended for mucosal drug delivery . Uppsala, 2003. 76 f. [Dissertation for the degree of Doctor of Philosophy in Pharmaceutics. Uppsala University]
13. Hagerstrom H, Edsman K, Stromme M. Low-Frequency Dielectric Spectroscopy as a Tool for Studying the Compatibility between Pharmaceutical Gels and Mucus Tissue. J. Pharm. Sci. , v.92, n.9, p.1869-1881, 2003.
14. Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv.Drug Del. Rev . , v.57, n.11, p.1556-1568, 2005.
15. Mathiowitz E, Chickering DE, Lehr CM. Bioadhesive drug delivery systems: fundamentals,novel approaches, and development. Drugs and thePharmaceutical Sciences . New York: Marcel Dekker,1999. 696 p.
16. Huang Y, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: Tetheres structures and site-specific surfaces. J. Control. Release , v.65, n.1, p.63-71, 2000.
17. Rahamatullah Shaikh, Thakur Raghu Raj Singh, Martin James Garland, A David Woolfson, Ryan F Donnelly. Mucoadhesive drug delivery systems. JBPS[online] 2011, vol 3, p.89-100
18. Flávia Chiva Carvalho, Marcos Luciano Bruschi, Raul Cesar Evangelista, Maria Palmira Daflon Gremião. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci., vol. 46, n.1, jan./mar., 2010, pp. 1-17
19. Peh KK, Wong CF. Polymeric Films as vehicle for buccal drug delivery: Swelling, Mechanical and bioadhesive properties. J Pharm Pharmaceutics Sci 1998; 2: 53-61.
20. Madhusudan Rao Y, Vani G, Bala RCR. Design and evaluation of mucoadhesive drug delivery systems. Indian drugs 1999; 35: 558-65.
21. Teng CL, Ho NF. Mechanistic studies in the simultaneous flow and adsorption of polymer-coated latex particle on intestinal mucus I: Methods and physical model development. J Control Release 1987; 6: 133-49.
22. Ranga Rao KV, Buri P. A novel in situ method to test polymers and coated micro particles for bioadhesion. Int J Pharm 1989; 52: 265-70.
23. Rao YM, Vani G, Bala R, Chary R. Design and evaluation of mucoadhesive drug delivery systems. Drug Dev Ind Pharm 1998; 35: 558-65.
24. Gandhi BR, Robinson JR. Bioadhesion in drug delivery. Ind. J.Pharma. Sci, 1988, 50, pp:145-152.
25. NK Jain. Controlled release and Novel Drug Delivery. 1st edition.CBS publishers and Distributors New Delhi.1997, 353-370.
26. D Chickering, J Jacob, E Mathiowitz.. Poly fumaric-cosebacic microspheres as oral drug delivery systems. Biotechnol. Bioeng. 1996, 52, 96–101
27. Jimenez - Castellannos NR, Zia H, Rhodes CT. Mucoadhesive drug delivery systems, Drug Dev. Ind Phar.19 142 , 1993, 143.
28. Longer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems, Biomaterials. 2 4 , 1981,201- 14.
29. Park K, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery: method to study bioadhesion. Int J Pharm, 19, 1984, 107–127.
30. Smart JD, Kellaway IW, Worthington HEC. An in vitro investigation of mucosa adhesive materials for use in controlled drug delivery. J Pharm Pharmacol, 36, 1984, 295-99.
31. R. K. Chang, A. J. Shukla. Polymethacrylates. In: Raymond CR, Paul JS, Paul JW, ed. Handbook of Pharmaceutical Excipients. The Pharmaceutical Press and The American Pharmaceutical Association; 2003: 462-468.
32. K. Hizawa, H. Otsuka, H. Inaba, et al. Subcutaneous pseudosarcomatous PVP granuloma. Am. J. Surg. Path., 1984, 8: 393-398.
33. J. J. Kolen, J. W. McGinity, W. R. Wilber. Carbomer–934P. In: Raymond CR, Paul JS, Paul JW, ed. Handbook of Pharmaceutical Excipients. The Pharmaceutical Press and The American Pharmaceutical Association; 2003: 89-92.
34. P. J. Weller, S. C. Owen. Polyvinylalcohol. In: Raymond CR, Paul JS, Paul JW, ed. Handbook of Pharmaceutical Excipients. The Pharmaceutical Press and The American Pharmaceutical Association; 2003: 491-492.
35. Al-T. Khaled, S. Jagdish. Recent patents on drug delivery and formulation. 2007, 1: 65-71
36. Agnihotri S.A., Mallikarjuna N.N., and Aminabhavi T.M., Recent advances on chitosanbased micro- and nanoparticles in drug delivery, J. Cont. Rel., 2004, 100 1 , 5–28.
37. Kheri R., and Agrawal J., Chitosan as classic biopolymer: a review, Inter. J. Pharm. and Life Sci., 1 7 , 369-372.
38. Christina T., and Stamford M., Growth of cunninghamella elegans UCP 542 and production of chitin and chitosan using yam bean medium, Elect. J. Biotech., 2007, 10 1 , 1-12.
39. Amorim V.D.S., Souza W.D., Fukushima K., and Takaki G.M.D.C., Faster chitosan production by mucoralean strain in submerged culture, Brazil. J. Microbio., 2001, 32 1 , 1-11.
40. Dhawan S., Singla A.K., and Sinha V.R., Evaluation of mucoadhesive Properties of chitosan microspheres prepared by different methods, Pharm. Sci. Tech. 2004, 5 4 , 1-12.
41. Ahn J.S., Choi H.K., and Cho C.S., A novel mucoadhesive polymer prepared by template polymerization of acrylic acid in the presence of chitosan, Biomaterials, 2001, 22 9 , 923-928.
42. Wong W.T., Chitosan and its use in design of insulin delivery system, Recent Patents on Drug Deliv. and Formu., 2009, 3, 8-25.
43. Rabea E.I., Badawy M.E.T., Stevens C.V., Smagghe G., and Steurbaut W., Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules, 2003,4 6 , 1457-1465.
44. Mansouri S., Lavigne P., Corsi K., Benderdour M., Beaumont E., and Fernandes J. C. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy – strategies to improve transfection efficacy, Euro. J. Pharm. and Biopharm., 2004, 57 1 , 1-8.
45. Tomolin, J., Taylor J.S., and Read N.W., The effect of mixed faecal bacteria on a selection of viscous polysaccharide in vitro, Nutr. Rep. Int., 1989, 39,121–135.
46. Bayliss C.E., and Houston A.P., Degradation of guar gum by faecal bacteria, Appl. Environ. Microbiol., 1986, 48,626–632.
47. Macfarlane G.T., Hay S., Macfarlane S., and Gibson G.R., Effect of different carbohydrates on growth polysaccharidases and glycosidase production of bacteroides ovatus in batch and continuous culture, J. Appl. Bacteriol., 1990, 68,179–187.
48. Dürig T., and Fassihi R., Guar-based monolithic matrix systems: effect of ionizable and nonionizable substances and excipients on gel dynamics and release kinetics, J. Cont. Rel., 2002, 80, 45-56.
49. Hagiwara A, Boonyaphiphat P, Kawabe M, Naito, H., Shirai, T., and Ito, N. Lack of carcinogenicity of tragacanth gum in B6C3F1 mice. Food Chem Toxicol1992; 30 8 : 673–679.
50. Roew, and Raymond , Adipic Acid, Handbook of Pharmaceutical Excipients, 2009 ,11–12
51. Remminghorst, U., Rehm, B.H.A., (2009) Microbial production of alginate: biosynthesis and applications. In Microbial Production of biopolymers and polymer precursors: Applications and perspectives. ed. Bernd. H.A. Rehm; Caister Academic Press, 2:13-42.
52. Handbook of Pharmaceutical Excipients: FIFTH EDITION 2006: Edited by Raymond C Rowe BPharm, PhD, DSc, FRPharmS, CChem, FRSC, CPhys, MInstP, 657
53. Handbook of Pharmaceutical Excipients: FIFTH EDITION 2006: Edited by Raymond C Rowe BPharm, PhD, DSc, FRPharmS, CChem, FRSC, CPhys, MInstP, 785
54. Sakr AM, Elsabbagh HM, Shalaby AH. Effect of the technique of incorporating sodium alginate on its binding and/or disintegrating effectiveness in sulfathiazole tablets. Pharm Ind 1978; 40 10 : 1080–1086.
55. Klaudianos S. Alginate sustained-action tablets [in German]. Dtsch Apoth Ztg 1978; 118 : 683–684. So di um Alginate 657
56. Holte O, Onsoven E, Myrvold R. Sustained release of water-soluble drug from directly compressed alginate tablets. Eur J Pharm Sci 2003; 20 4–5 : 403–407.
57. Azarmi S, Valizadeh H, Barzegar JM, Loebenberg R. ’In situ’ cross-linking of polyanionic polymers to sustain the drug-release of acetazolamide tablets. Pharm Ind 2003; 63 9 : 877–881.
58. Veski P, Marvola M, Smal J,et al . Biopharmaceutical evaluation of pseudoephedrine hydrochloride capsules containing different grades of sodium alginate. Int J Pharm 1994; 111 : 171–179.
59. Zatz JL, Woodford DW. Prolonged release of theophylline from aqueous suspensions.Drug Dev Ind Pharm1987; 13: 2159–2178.
60. Bodmeier R, Wang J. Microencapsulation of drugs with aqueous colloidal polymer dispersions. J Pharm Sci1993; 82: 191–194.
61. Rajaonarivony M, Vauthier C, Couarraze G, et al . Development of a new drug carrier made from alginate. J Pharm Sci 1993; 82 9 : 912–917
62. Sutton A., Harrison G.E., Carr T.E., and Barltrop D., Reduction in the absorption of dietary strontium in children by an alginate derivative, Br. J. Radiol., 1971, 44 523 , 567.
63. Sutton A., Harrison B. E., Carr T. E., and Barltrop D., Reduction in the absorption of dietary strontium in children by an alginate derivative, Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 1971, 19 1 , 79-85.
64. Vennat B, Lardy F, Arvouet-Grand A, Pourrat A. Comparative texturometric analysis of hydrogels based on cellulose derivatives, carraghenates, and alginates: evaluation of adhesiveness. Drug Dev Ind Pharm 1998; 24 1 : 27–35.
65. Miyazaki S, Nakayama A, Oda M, et al . Drug release from oral mucosal adhesive tablets of chitosan and sodium alginate. Int J Pharm 1995; 118 : 257–263.
66. Attia MA, ElGibaly I, Slialtout SE. Transbuccal permeation, anti-inflammatory and clinical efficacy of piroxicam formulated in different gels. Int J Pharm 2004; 276 : 11–28.
67. Mohammed FA, Kheder H. Preparation and in vitro/in vivo evaluations of the buccal bioadhesive properties of slow-release tablets containing miconazole nitrate. Drug Dev Ind Pharm2003; 29 3 : 321–337.
68. Rao P. S. Shrivastava HC. Industrial Gums . 5th ed. New York : Whistler RC; 1973.
69. Rao KH, Subramanian N. Protein foods feeds. Proc Matt Symp. 1984; A67-A87.
70. Bhattacharya S, Bal S, Mukharjee RK, Bhattacharya S. Rheological behavior of tamarind kernel powder suspension. J Food Eng. 1991; 13: 151-58.
71. Saetone MF, Burgalassi S, Giannaccini B, Bodrini E, Bianchini P, Luciani G. Ophthalmic solutions viscosified with Tamarind seed powder. PCT, Int Appl WO9728. 1997.
72. Kulkarni D. Dwivedi D. K., Sarin JP, Singh S. Tamarind seed polyose: A potential polysaccharide for sustained release of verapamil hydrochloride as a model drug. Indian J Pharm Sci. 1997; 59 1 : 1-7.
73. Scholars Research Library, Der Pharmacia Lettre, 2010, 2 4 : 429-431, Evaluation of binder’s efficiency of different natural gums in tableting process Braja B. Panda, Debasis Mishra, Goutam Ghosh, P. Sudhir Kumar and Puspita Acharya
74. Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy, 30th ed. 2005; Nirali Prakashan, Pune.
75. ScienceAsia 36 2010 : 319–325 Mucoadhesive beads of gliclazide: Design, development, and evaluation Amit Kumar Nayak, M. Saquib Hasnain, Sarwar Beg, M. Intakhab Alam
76. Research in Pharmacy 1 4 :10-16, 2011 ISSN : 2231-539X, Formulation and evaluation of a novel mucoadhesive drug delivery system to treat intestinal candidiasis in immunocompromised patients SuparnaDugal and Andrea Fernandes
77. Nigerian Journal of Pharmaceutical Sciences Vol. 7, No. 1, March, 2008, ISSN: 0189-823X, STUDIES ON SOME PHYSICOCHEMICAL PROPERTIES OF KHAYA SENEGALENSIS GUM Mahmud, H S., Oyi, A.R and Allagh, T.S
78. Odeku OA, Fell JT. Evaluation of khaya gum as a directly compressible matrix system for controlled release, J. Pharm. Pharmacol 2004;56:1365–70.
79. Odeku OA, Fell JT. In-vitro evaluation of khaya and albizia gums as compression coatings for drug targeting to the colon. J Pharm Pharmacol 2005; 57 2 :163-68.
80. Trease GE, Evans MC, editors. Textbook of Pharmacognosy, 15 th ed., Balliere, Tindall: London; 2002.
81. Avachat A, Gujar KN, Kotwal VB, Patil S. Isolation and evaluation of fenugreek seed husk as a granulating agent. Indian J Pharm Sci 2007;69:676-9









