{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Poonam R. Ithape1*, Priyanka D. Ghadage1, Jayant K.Gadhve1,Akash S.Mali
Gourishankar Institute of Pharmaceutical Education and Research,
Limb, Satara, Maharashtra
*poonamithape6996@gmail.com
ABSTRACT
The focus of present study is to exist a novel methods for pediatric oral drug delivery.To overcome the problems associated with drug administration,toxicity and taste preference the drug designed in such way that it best suits to child age,required treatment,size and physiological condition.Pediatrics differs from adult in many aspects of pharmacotherapy. Palatability is the biggest challenge while designing the pediatric formulation. As the vast no of limitation present in solid formulation there is wide scope in novel research in designing the pediatrics drug delivery system. Novel approaches in oral drug delivery system is somewhat satisfy the challenges but not the give proper solution. To overcome these problems the novel innovativeresearches is going on. The formulation should be designed in such a way that is easy to administer for pediatrics,having dose flexibility and better palatability of drug formulation.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2588
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 6 Received On: 10/03/2018; Accepted On: 24/04/2018; Published On: 01/06/2018 How to cite this article: Ithape P, Ghadage P, Gadhve J, Mali A; Oral Drug Delivery System Challenges to Pediatrics and Current Approaches; PharmaTutor; 2018; 6(6); 9-13; http://dx.doi.org/10.29161/PT.v6.i6.2018.9 |
INTRODUCTION
Oral drug delivery system can be very challenging for pediatrics, throughout continuous growth period[Anne cram et al.,2009 ]. The novel drug delivery system design to prevail the problems affiliated with physiological impairment and swallowing.Evolution of oral drug delivery system extremely challenging forthy wide range of pharmaceuticals and clinical aspects regarding quality, safety, efficacy of developed formulation. Demands, needs and special quality of pediatrics formulation make them challenging to develop it. Pharmacokinetic and pharmacodynamic parameters of oral drug alters due to various growth stages of pediatrics which requires dose flexibility which ensembles all age groups of pediatrics dosing requirement. Oral formulations are reasonable and convenience for the patients. Pensively all views show that pediatric formulation having various approaches to novel technologies, designing and development since consequently fastest innovation occurs in this area.
In these field of pediatric drug delivery system some groups continue to guide research and champion technologic advances.In 2005 Eunice Kennedy Shriver National Institute for child health and human development joined by representatives from pharmaceutical industry,academic medicine,and from Food and Administration(FDA),formed the United states(US)Pediatric Formulation Initiative to provoke the research in pediatric formulation technology.
A likely European Medication Agency (EMA) develops the European Pediatric Formulation Initiative. In 2007 World Health Organization (WHO) launched a global initiative titled as “Make Medicines Child Size” to novel development of pediatric dosage form.
Recent Methods and Development:
Liquid dosage form
Due to broad range of limitation of liquid dosage form over solid dosage form in this era scientist move towards development of solid dosage form. In some cases, liquid dosage forms are also acceptable such as, infants neonats due to increase dose flexibility and ease of swallowing over a solid dosage forms.Liquid dosage forms need to administer multiple doses throughout the day due to lack of development of control release dosage formNumber of research have been investigated for the development of sustain release liquid such as, ion exchange resin, coated micro particles in suspension and among other but the success of this is not maintained only few sustained release formulation are available in market.
Appropriate vehicle is important in pediatric formulation with improve palatability. Now a day’s milk has been explored as a vehicle for liquid formulation showing high solubility and stability [ Mishra B et al.,2011].
Also the lipid based vehicle are provide solubility of highly lipid soluble drug. The developing field in liquid dosage form is development of administration devices such as baby bottle coupled to a syringe for administration of liquid dosage forms.
Solid dosage form
As the vast number of limitation solid dosage forms are superior to the formulation of pharmaceutical industry. It provides long term stability, easing supply chain and low manufacturing cost. Convential solid dosage forms are not largely acceptable to pediatric patients due to swallowing difficulties and flexible dose[Felipe L Lopez et al.,2015 ]. In such cases administrating device such as “pill swallowing cups” has been used to increase suitability of administration.In the recent era interesting development of solid dosage form is “solid dosage pens”[ Mishra B et al.,2011] it is pen like device which adjust the dose by cutting the tablet into small slices of the required length.Recent development of new packaging system which improve the both stability, safety and acceptability of dosage forms.Compliance prompting packaging include printed blisters which provide self monitoring treatment and guidelines for correct administration of medication.
Fig No .1 Era of developing Pediatric drug delivery system
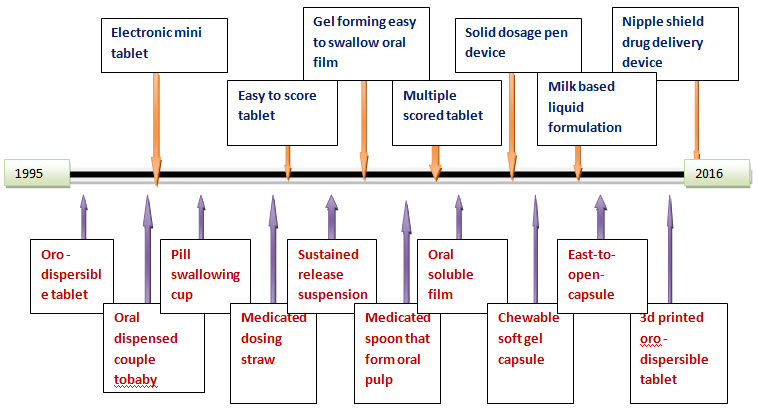

Novel approaches in oral drug delivery system in pediatrics
1) Oriodispersable tablets:
ODT’S developed to exclude the problem regarding swallowing of whole tablet which is sometimes not tolerated by pediatrics and which disintegrates easily within fraction of second. “Fast dissolution and Fast disintegration” property helps in designing of such novel methods it also eliminates the role of vehicles like water, milk.[4] The major advantages of ODT’S over tablet is that no need of water, dose pliability and swallowing is avoided. It also offers better onset of action and bioavailability. Regarding to fragility of ODT formulations the tablet splitting defect ordinarily contraindicated which may affects dose flexibility .To overcome such problems “orally disintegrating mini tablets” are used. The major advantage of such formulations it offers combined assistance of ODT’S and multiparticulates. The absorption of drugs mostly via gastrointestinal tract and alternatively sublingual and buccal which offers advantage for onset of action and bioavailability. Lyophilisation, direct compression,3D printing technology, tablet molding, flash heat method are the certain approaches for developing ODT’S. Manufacturing of ODT’S highly controlled by patented technologies. Recent ODT platform based on3D printing technology with advantages high drug loading and very rapid disintegration. Examples of drugs available in market as ODT’S Olanzapine, Risperidone, Selegiline, Tramadol, Donepezil, Lamictal [Marcia L.Buck,2013 ].
Oriodispersable films:
ODF’S designed to develop quick disintegrating preparations is based on polymeric matrix. Possess certain advantages no need of swallowing, no need of water, improvement in bioavailability by buccal absoption, Continuous manufacturing achieved. And limitations such as control release and taste masking is challenging, uniformity of dose also challenging, specialized packaging required. Moreover it possess elegant appearance, increased flexibility of dose, as a different strengths achieved by cutting the films of required size. The fast dissolving advantage is not for purposeful activity anymore as they designed to fast adherence to buccal mucosa and to obtain drug release in timely manner. Typically it is manufactured by solvent casting method composed of polymeric matrix with embedded drug .Alternative method is hot –plate –extrusion where solvent use is avoided beneficial for control release and taste masking of films .In the developing era the Novel methods arising such as electrospinning ,Ink jet printing beneficial for higher drug doses >100 mg amount is limited thus only potent drug with specific physicochemical property can easily administered. For improvement of stability and for reducing risk of overdosing ODF’S sealed individually which reduces chances of film sticking. It normally presents to patient in form of stamp –like strips either single dose sachets or multidose sachets.
3) Multiparticulate drug delivery system :
Multiparticulate drug delivery system designed to provide improved acceptability of patient over convectional dosage forms (i.e tablet, capsule) [Cunamvilla jaltojs and torres D,2000 ].It composed of number of discrete units such as pellets, granules, minitablets. Moreover it usually suitable for controlled release and taste masking. possess certain advantages Ease of functionalisation, Excellent flexibility of dose, Administration flexibility, Easy availability of manufacturing technology, Targeted release profile, suitability of taste masking, Bioavailability is highly reproducible, due to reduced size swallowing is aided. Limitations are Grittiness and mouth feel, Altered Bioavailability due to co-administration with food/drinks. Needs special administration equip mentor accessories, Need to develop packaging /dosing platform, Food drug compatibility needs to be studied. For the administration of multiparticulate formulations oral gels and in situ gelling vehicles are being studied for aid. Milk, applesauce, water are potentially used vehicles for such formulations.
Recent studies stated that for pediatric formulation mostly yogurt and milk is suitable. The manufacturing process usually includes polymeric coating step as downstream processing for improvement is control release and taste masking property. Regarding to presentation and packaging it offers high degree of flexibility. For ease of use to patient the formulations can be filled into capsules. Furthermore granules and pellets can also be incorporated into medical devices for better administration .Example of administration device that is dose sipping technology, medicated spoons. Generally volumetric spoons having cost effective approach although the accurate dosing is limited.For better and high accurate dosing more sophisticated devices are needed. Overall these technologies may be more costly to develop and design.
Chewable formulations:
Chewable formulations (soft chews, chewable tablet, chewing gums)designed to promote better disintegration and dissolution of API. These formulations usually prepared by use of hydrophilic sweetners like Mannitol which offers good mouth cooling taste, Seize certain advantage swallowing avoided, No need of water, may be preferred over convectional formulation, Bioavailability may be improved by buccal absorption ,Availability of manufacturing and packaging technology. Need of chewing limits the use of such formulations in pediatrics it can be well tolerated by children’s of age 2 years. Possess certain limitation Taste masking and control release is challenging, various dosage strengths may required, May need of specialize accessories and devices(equipments).Chewable tablets typically prepared by compression method similar to as ODT’S but disintegrators are not used in these formulations. In addition these formulations may be portended by patients rather than using other formulations cause of its wide range of esthetic properties. There are also some patented technologies for manufacturing and preparation of chewable dosage formulations .For example Paulsen method based on tablet molding where water use and high temperature is avoided. Other one technology based on soft gelatin capsule technology modified by using chewable filters .It offers the benefit of soft gel while avoiding need of whole swallowing of capsule.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Challenges
Palatability of age appropriate oral medication is central for attachment to therapeutic control therefore buildup of palatable formulation facing various challenges.
1) Challenges related to delicacy valuation:
While developing pediatric formulation priority given to the delicacy of the formulation. Never ever taste of the active pharmaceutical ingredient (API) will recognized by the patient while administration therefore its challenging task to mask taste of API in formulation. One of the extreme disadvantage of delicacy valuation is in vitro determination is not possible. The sagacity of delicacy of medication is different in adult and in pediatrics and also differs in healthy child and ill child. Therefore clinical study done on only children but some principles issue occurs to clinical trials. That’s why ‘swill and spit’ method good for delicacy valuation. But in some cases the healthy volunteers are not used ethically also ‘swill and spit’ methods. For e.g. testing of drug cytotoxics is consider as unethical for healthy volunteers. In such cases it is difficult to study the palatability and acceptance of drug by pediatrics[Anne cram et al.,2009].
In other way its valuation can be done while prescribing medication frequently to patients rather than in sing dose administration. In practice due to lack of testing assessment technique difficulties in formulation occurs. Problems of delicacy valuation can also be solved by taking consideration of flavors preferred by Childs such as cherry flavor, chocolate, apple ,strawberry that added to liquid formulation may increases the acceptance of formulation
2) Challenges related to medicine and formulation improvement:
During improvement of novel used for oral administration the main significance is on relevant formulation property for adult dosage form with the intention of developing of conventional dosage form. Where the younger children and older children preferably used oral solid dosage form and smaller than this prefer liquid dosage form.
While developing formulation taste is not considered as the priority rather than physical and physicochemical parameters on the top. While developing the pediatric formulation solubility characteristics of drug is highly tasking property. when drug having highly solubility characteristic is difficult to mask the taste of that compound and formulate a liquid dosage forms such as suspension. Because it get solubilized easily in vehicle. taste masking in solid formulation such as chewable tablet and oriodispersible tablets is also difficult due to highly solubility of drug and easily dissolve in mouth .alternative of such condition coating of drug and formulation of film coated mini tablets and pellets is carried out.
CONCLUSION
The development of pediatric pharmaceutical products is associated with considerable challenges including physiological impairment and swallowing and also combine demands of industry, patients and healthcare providers. Many pediatric formulations available now a day but they are not fulfilling the needs of patient and manufacturers too for thy further development is going on the novel methods for designing of pediatric formulation. During the past two decades numbers of pediatrics formulations have been investigated, developed and patented. The current strategies for the formulation of pediatrics medicine have been reviewed throughout this article i.e some novel formulations like ODT’S,ODF’S, Multiarticulate drug delivery system which helps in improvement regarding the pediatric formulations the patient needs, acceptance, manufacturer’s needs .The stability and ability of such novel formulations helps in designing of pediatric dosage forms as per expectation .These innovations may leading to new alternatives which better suited to pediatrics. As stated in manuscript the non commercial technologies are the area for development in pediatric medications. However some new novel areas have been identified where further research may accelerate the novel pediatric formulation development process.
REFERENCE
1. (Anne cram et al.,2009)Challenges of developing palatable oral pediatric formulation ;Int j Pharm 365 1-3)
2. (BatchelorHK et al.,2014) Pediatric oral Bio pharmaceutics: Key considerations and current challenges.Adv Drug Delivery Rev ;73:102—26
3. (Cuna m villa jaltojs and torres D,2000) control release liquid suspension based on ion exchange particle entrapped within acrylic microcapsule ; Int j pharm ;199(2);158-8.
4. (Ivanovska V et al.,2014)Pediatric drug formulation :a review of challenges and progress;134(2):361--72
5. (Felipe L Lopez et al.,2015) formulation approaches to pediatric oral drug delivery; benefit and limitation of current platform; expert opinion drug delivery Nov 2;12(11);1727-1740
6. (Kraus dm et al .,2001) Effectiveness and infant acceptance of the Rx medibottel versus the oral syringe. pharmacotherapy :21(4); 416-23
7. (Marcia L.Buck,2013) Pharm.D, FCCP,FPPAG Alternative forms of oral drug delivery for pediatric patient.
8. (Matsui,D,2007).Current issues in pediatric medication adherence. Paediatric. Drugs 1.9,283-288
9. (Meltzer Eo et al.,2016) Pill swallowing ability and training in children 6 to 11 year old age. Clinpediatr :45(8):725-33.
10. (Mishra B et al.,2011).design of control release liquid formulation of lomotrigim. DARU J pharmacist ;19(2)
11. (Van Riet-Nales DA et al.,2010) Effects of the pharmaceutical technological aspects of oral pediatric drugs on patient-related outcomes : a systemic literature review. Clin Ther :924—38
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









