{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Bhushan M. Firake*, Ranjini Chettiar, Tejal B. Firake
Depertment of Pharmaceutical Analysis,
JSPM’s Jayawantrao Sawant College of Pharmacy & Research,
Hadapar, Pune.
*bmf.jscopr@gmail.com
ABSTRACT: Nitazoxanide is an antiprotozoal and anthelmintic agent, which is mostly used in the treatment and prevention of wide variety of protozoa, helminthes and gram negative organisms. This article studies published analytical techniques that are reported so far for the determination of nitazoxanide in bulk, pharmaceutical formulation and biological samples. They include various techniques like spectrophotometry, electrochemical methods, capillary electrophoresis, high performance liquid chromatography, high performance thin layer chromatography, and liquid chromatography-mass spectrophotometry.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2516
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 9 Received On: 02/05/2017; Accepted On: 12/05/2017; Published On: 01/09/2017 How to cite this article: Firake BM, Chettiar R, Firake TB;Nitazoxanide: A Review of Analytical Methods; PharmaTutor; 2017; 5(9);61-68 |
INTRODUCTION:
Nitazoxanide (NTZ) is a new antiparasitic and antiprotozoal agent having broad spectrum of activity. It is a nitrothiazole derivative and its chemical name is 2-acetyloxyl-N-(5-nitro-2-thiazolyl) benzamide (Fig. 1) (1). It was initially developed as a veterinary anthelminthic with activity against intestinal nematodes, cestodes and trematodes. NTZ was approved by the US Food and Drug Administration (FDA) in 2002 for use in human beings (2). It is used for treating both intestinal protozoal infections and helminthiasis (3). It is also used for treating diarrhea caused by Giardia lamblia as well as for cryptosporidiosis in immune-compromised patients, including those with AIDS or HIV infection (4, 5, 6, 7, 8). The antiprotozoal activity of NTZ is believed to be due to interference with the pyruvate ferredoxin oxidoreductase (PFOR) enzyme dependent electron transfer reaction which is essential to anaerobic energy metabolism. Studies have shown that the PFOR enzyme from Giardia lamblia directly reduces nitazoxanide by transfer of electrons in the absence of ferredoxin. The DNA-derived PFOR protein sequence of Cryptosporidium parvum appears to be similar to that of Giardia lamblia. Interference with the PFOR enzyme-dependent electron transfer reaction may not be the only pathway by which nitazoxanide exhibits antiprotozoal activity. NTZ is a light yellow/ pink crystalline powder which is insoluble in water and poorly soluble in ethanol. It has a molecular mass of 307.283 g/mole and molecular formula of C12H9N3O5S (9). After ingestion, it is converted to the active metabolites tizoxanide and tizoxanide glucuronide. In plasma, more than 99% of NTZ is bound to proteins. It is available in the market as tablets and oral suspension.
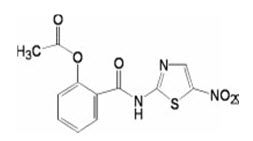
Fig. 1: Structure of Nitazoxanide
SOLUBILITY PREPARATION:
Solubility:
According to Biopharmaceutical Classification System (BCS), nitazoxanide is a class IV drug (low solubility and low permeability).It is slightly soluble in acetone, choloroform and very slightly soluble in methanol and practically insoluble in water. The melting point of nitazoxanide is 202°C. (10)
Sample preparation strategies:
Sample preparation is the integrated part of analytical methodology, and it was reported that approximately about 30% errors contributed from sample analysis was due to sample preparation. Various diluents used for the analysis of nitazoxanide include acetonitrile:0.2M potassium dihydrogen phosphate 70:30 (pH 3.0 adjusted with o-phosphoric acid), acetonitrile; 0.3M potassium dihydrogen phosphate:methanol 70:10:20 (pH 3.5 adjusted with o-phosphoric acid), 1N hydrochloric acid, methanol, ethanol, sodium hydroxide, ammonia acetate, glacial acetic acid, acetonitrile, chloroform, ammonia solution. Solvent used are 1, 4-dioxane, dimethyl formamide (DMF), acetonitrile, ethanol. The sample preparation technique for the extraction of nitazoxanide from the biological matrices like plasma, serum, urine, liver, kidney and brain was by deproteination with acetonitrate, ethanol and followed by centrifugation.
ANALYTICAL METHODS:
Spectrophotometry: In the literature, 9 methods were reported for the estimation of NTZ using spectrophotometry, of which 7 methods are for determining NTZ alone, whereas the remaining are for quantifying NTZ in combination with other drugs substance. Table 1 shows the summary of the reported spectroscopic methods indicating the basic principle, λ max, solvent, limit of detection (LOD) and limit of quantification (LOQ).
|
Compounds |
Method |
λ max (nm) |
Solvent |
LOD (µg/ml) |
LOQ (µg/ml) |
Ref. |
|---|---|---|---|---|---|---|
|
NTZ |
Hyposchromic shift based method |
343.5 |
Methanol:0.1M citric acid (80:20) |
0.12 |
0.39 |
11 |
|
NTZ in dosage form |
Spectrophotometry |
238.3 |
Acetonitrile:water(9:1) |
12 |
||
|
NTZ |
Spectrophotometry |
732 |
1ml ferric chloride (1%) and 2ml MBTH (0.1%) |
0.1147 |
0.3824 |
12 |
|
NTZ |
Simultaneous Equations Method |
218.5 |
Ferric chloride |
0.7653 |
0.8796 |
13 |
|
NTZ |
First derivative spectroscopy |
277 |
Ferric chloride |
1.2374 |
1.1134 |
|
|
NTZ |
Second derivative spectroscopy |
260,314 |
Ferric chloride |
1.6543 |
1.2467 |
|
|
NTZ |
spectrophotometry |
344 |
ethanol |
0.907 |
0.299 |
14 |
|
NTZ, Ofloxacin |
Q-analysis method |
346.36 |
1NHCl in methanol |
15 |
||
|
NTZ, Ofloxacin |
Vierodts method |
346.3,296.49 |
1N HCl in methanol |
15 |
||
|
NTZ, Ofloxacin |
Dual wavelength method |
333.6, 359.2, 302.4, 289.2 |
1N HCl in methanol |
15 |
Electrochemical methods: The determination of electrochemical behavior of NTZ was studied using voltametry.The authors used hanging mercury electrode as sensor for the NTZ in Britton-Robinson universal buffer of pH values 2 to 11.Methods used for voltammetric determination are cyclic and square-wave voltammetry,Cyclic voltammetric Square wave cathodic adsorptive stripping voltammetry (SW-CAdSV) and Differential pulse cathodic adsorptive stripping voltammetry (DP-CAdSV), linear sweepcathodic adsorptive stripping voltammetry (LS-CAdSV),differential pulse polarography (DPP).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
|
Compounds |
Method |
Linear response |
Correlation coefficient |
LOD (µg/ml) |
LOQ (µg/ml) |
Ref. |
|---|---|---|---|---|---|---|
|
NTZ in pharmaceutical formulation |
cyclic and square-wave voltammetry |
20–140 mg/mL |
0.9938 |
5.23 μg/mL |
17.45 μg/mL |
16 |
|
NTZ in human serum |
LS-CAdSV DP-CAdSV SW-CAdSV |
3×10-9 to 2×10-7 mol L-1, 5×10-9 to 1×10-7mol L-1, 1×10-9 to 1×10-7mol L-1 |
0.985, 0.990, 0.999 |
9x10-10, 1.5×10-9, 3×10-10mol L-1 |
3×10-9, 5×10-9, 1×10-9mol L-1 |
17 |
|
NTZ in human urine |
DP-CAdSV, SW-CAdSV |
1×10-9 to 1×10-8mol L-1 |
0.9965, 0.9985 |
2.078×10-10, 1.365×10-10 mol L-1 |
4.551×10-10, 6.926×10-10 mol L-1 |
18 |
|
NTZ in human breast milk |
DP-CAdSV, SW-CAdSV |
1×10-8 to1×10-9 mol L-1 |
0.9999, 0.9993 |
0.601×10-10 mol L-1, 0.718×10-10 mol L-1 |
2.00×10-10 mol L-1, 2.393×10- 10 mol L-1 |
18 |
|
NTZ in bulk form |
DP-CAdSV, SW-CAdSV |
1x10-9 to 1x10-8 mol L- 1 |
0.9961, 0.999 |
1.878x10-10 mol L-1, 1.078x10-10 mol L-1 |
of 6.262 x10-10 mol L-1, 3.595x10-10 mol L-1 |
18 |
Shital Gandhietal(19)developed a simple, sensitive and highly selective electrochemical method for the simultaneous determination of NTZ and ofloxacin in aqueous media (Britton-Robinson buffer, pH-8.36) on a hanging mercury drop electrode (HMDE) using differential pulse polarography (DPP). Using DPP a separation of about 936 mV between the peak oxidation potentials of nitazoxanide and ofloxacin present in binary mixtures was obtained. The quantification limits for the simultaneous determination of NTZ and ofloxacin were 0.083 μg/ml and 0.208 μg/ml.
Chromatography;
HPLC:
1. Biological samples:
A high-performance liquid chromatographic method was optimized and validated for the determination of desacetylNTZ (tizoxanide), the main active metabolite of NTZ in human plasma, urine and breast milk by Ghada M.etal(20). The proposed method used a CN column with mobile phase consisting of acetonitrile–12mM ammoniumacetate–diethylamine in the ratio of 30:70:0.1 (v/v/v) and buffered at pH 4.0 with acetic acid, with a flow rate of 1.5 mL/min. Quantitation was achieved with UV detection at 260 nm using nifuroxazide as internal standard.
Human urine sample was prepared by alkalizing with 0.1M sodium hydroxide sonicated for 15 min and neutralized with 0.1M hydrochloric acid. The LOD was found to 3.56 _ 1022be and LOQ was found to be 11.87 _ 1022.
Human breast milk sample was prepared by homogenizing and mixing with nifuroxazide and orthophosphoric acid. The LOD was found to be 4.47 and LOQ was found to be 14.9.
Human plasma was preparedby mixing with acetonitrile, NF 0.1M sodium hydroxide. It is sonicated for 15 min., neutralized by 0.1M hydrochloric acid. The LOD 4.8 was found to be and LOQ was found to be 16.24.
2. Pharmaceutical samples: Analytical methods for the determination of isoniazid inpharmaceutical dosage forms using HPLC
|
Mobile phase |
Column |
Detection |
λ max (nm) |
Flow rate |
LOD |
LOQ |
Ref. |
|
Study aim: Quantification of NTZ in presence of its alkaline degradation product |
|||||||
|
acetonitrile: 50 mM ammonium acetate buffer (50:50, v/v, pH 5.0 adjusted with acetic acid) |
Inertsil C8-3 column (150 × 4.6 mm i.d.) |
UV |
298 nm |
1 mL/min. |
0.0410 |
0.1242 µg/mL |
21 |
|
Study aim: Isocratic reverse phase high pressure liquid chromatographic Method for the simultaneous determination of NTZ and Ofloxacin from combined dosage form |
|||||||
|
2.0gm sodiumdihydrogen phosphate and 5M of triethylamine are mixed into 500mL Milli Q water and pH was adjusted to 4.5 by orthophosphoric acid |
Phenomenex Luna C18 reversed-phase column |
UV |
305 nm |
1.5 mL/min |
0.174μg/ml and 0.21μg/ml |
0.034μg/ml and 0.08μg/ml |
22 |
|
Study aim: Simultaneous determination of NTZ and ofloxacin |
|||||||
|
Tetrabutyl ammonium hydrogen sulphate: methanol: acetonitrile (20:20:60) |
RP C-18 column (Shimadzu liquid chromatograph LC-10ATVP) |
UV |
282nm, 278 nm |
0.5ml/min |
0.0001306, 6.183 |
0.0001306, 6.183 |
23 |
|
Study aim: Simultaneous determination of NTZ and ofloxacin in pharmaceutical preparation. |
|||||||
|
acetonitrile: 0.2M potassium dihydrogen phospate in ratio 70:30 (pH 3.0 adjusted with orthophosphoric acid) |
Hypersil BDS C8 column (5μ particles size) (250 mm X 4.6 mm) |
UV |
319nm |
1.0ml/min |
- |
- |
24 |
|
Study aim: Determination of NTZ in oral suspension dosage form |
|||||||
|
mixture of acetonitrile: ammonium dihydrogen phosphate buffer (0.075 M) in the ratio 45:55 (% v/v) adjusted to pH 3.0 with orthophosphoric acid |
Qualisil BDS C18 (4.6 × 250mm, 5μ) |
UV |
240 nm |
1.5 mL/min |
0.25 |
0.77 |
25 |
|
Study aim: Simultaneous Estimation of NTZ and Ofloxacin from Tablet Dosage |
|||||||
|
acetonitrile: potassium dihydrogen ortho phosphate (pH 4.5, 10mM) (60:40 v/v) |
Princeton SPHER C18 column (250mm×/4.6 mm i.d.) |
UV |
265nm |
1.0ml/min |
|
|
26 |
T. Sakamoto et al(27) developed a simple and rapid determination method for NTZ using reverse-phase HPLC and Ultra Performance Liquid Chromatography (UPLC).
Mobile phase consisted of a mixture of phosphate buffer (pH 6.0) and acetonitrile HPLC System-HPLC Shimadzu Class-VP HPLC system. A Waters symmetry C18 Column (150 mm_4.6 mm I.D., 5 mm particle size, Waters Co., Milford, MA, USA),
The UPLC SYSTEM- A waters ACQUITY UPLC system. C18 (50 mm_2.1 mm I.D., 1.7 mm particle size, Waters Co., MA, USA)
The tailing factor of the NTZ peak was 2.0 for HPLC and 1.2 for UPLC, respectively. The retention times of IS (nifuroxazide) and NTZ were 22.1 and 24.8 min for HPLC, and 3.2 and 3.5 min for UPLC, respectively. The correlation coefficients were 0.9988 (HPLC) and 0.9963 (UPLC). The RSDs of quantitative values of sample solution were calculated to be 4.06% to 4.64% for HPLC and 0.15% to 0.36% for UPLC.
GC: The residual solvents in NTZ was developed by Jiang Shanet al(28) were separated by a DM-WAX column(30 m×0.25 mm,0.5μm) with an FID detector.The injector temperature and the detector temperature was set at 200℃and 250℃,respectively.The containers of head-space injector were in equilibrium at 80℃for 30 min.N,N-Dimethylformamide was used as the solvent.The detected solvents were separated completely.A good linearity of the two solvents was obtained within the range ol 250-750μg/ml(r=0.9991) and 30-90μg/ml (r=0.9991),respectively.The average recovery of acetone and dichloromethane was 99.15%and 99.18%with RSD of 2.17%and 2.97%(n=9),respectively.
LC-MS:
LC-MS method was developed by Zhanzhong Zhao et.al (29) developed a sensitive and specific method for the identification of NTZ metabolites in goat feces by liquid chromatography–electrospray ionization tandem mass spectrometry with negative ion mode was developed. After extraction procedure the pretreated samples were injected on an XTerra MS C8 column with mobile phase (0.2 mL min−1) of acetonitrile and 10 mM ammonium acetate (adjusted to pH 2.5 with formic acid) followed by a linear gradient elution, and detected by MS–MS. Identification and structural elucidation of the metabolites were performed by comparing their retention times (R t ), full scan, product ion scan, precursor ion scan and neutral loss scan MS–MS spectra to those of the parent drug or other available standard. The parent drug (NTZ) and itsdeacetyl metabolite (tizoxanide) were found in goat feces after the administration of a single oral dose of 200 mg kg−1 of NTZ. Tizoxanide was detected in goat feces for up to 96 h after ingestion of NTZ
Huang Xet al(30) utilized a hybrid linear ion trap/Orbitrap mass spectrometer providing a high mass resolution and accuracy was used to investigate the metabolism of NTZ in rats, pigs, and chickens. The results revealed that acetylation and glucuronidation were the main metabolic pathways in rats and pigs, whereas acetylation and sulfation were the major metabolic pathways in chickens, which indicated interspecies variations in drug metabolism and elimination. With the accurate mass data and the characteristic MS(n) product ions, we identified six metabolites in which tizoxanide and hydroxylated tizoxanide were phase I metabolites and tizoxanide glucuronide, tizoxanide glucose, tizoxanide sulfate and hydroxyl tizoxanide sulfate were phase II metabolites. Hydroxylated tizoxanide and tizoxanide glucose were identified for the first time. All the comprehensive data were provided to make out the metabolism of NTZ in rats, pigs and chickens more clearly.
The photodegradation of NTZ was studied byM.D. Malesuiket al(31) in order to investigate the degradation kinetics of this drug. The analyses of the degraded samples were performed by a stability-indicating liquid chromatographic method. The column utilized was aPhenomenex (Torrance, CA) Synergi Fusion C18 column (250mm, 4.6 mm, i.d., 4μm particle size) coupled to a C18 guardcolumn (4.0mm, 3.0mm, i.d., 4 μm). a mobile phase of ophosphoric acid 0.1% (v/v) (pH 6.0 adjusted by addition of triethylamine)– acetonitrile (45:55, v/v) run at a flow rate of 1.0mL/min and using PDA detection at 240 nm. The light source was an UVC – 254 nm30Wlamp (Philips, Amsterdam,Holland) fixed to a chamber in a horizontal position.
Degradation Rate Constant (k), Half-life (t1/2), and t90 for NTZ in Pharmaceutical Formulations Solutions Submitted to Photodegradation and Determined by LC Method.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
|
Dosage forms |
k/min |
t1/2 (min) |
t90 (min) |
|
Tablets |
4.81,10–2 |
201. 56 |
40.30 |
|
Powder for oral suspension |
5.38, 10–2 |
183.83 |
36.75 |
HPTLC:
A new simple, rapid, and selective high-performance thin-layer chromatographic (HPTLC) method with metronidazole as the internal standard has been developed by Salvador Namuret al(32) for analysis of tizoxanide (a metabolite of nitazoxanide) in human plasma. The analyte was extracted from human plasma by cation-exchange solid-phase extraction (SPE). In HPTLC the stationary phase was silica gel 60F254 and the mobile phase was toluene-ethyl acetate-acetic acid 6.2:13.4:0.4 ( v/v ). UV detection and quantification were performed at 313 nm for the internal standard and 410 nm for tizoxanide. Data were fitted to a quadratic mathematical function by polynomial regression. The working range was 400–16000 ng mL −1. The method was validated for accuracy and precision. The average recovery was 85.5%.
A validated stability indicating HPTLC was developed by CL Gopuet al(33) for determination of nitazoxanide in bulk and in formulation. They carried out separation in TLC alumina plates precoated with silica gel 60F254using mixture of ethyl acetate-toluene-methanol (3.9:6.1:1 and 4.1:5.9:1 v/v/v) as mobile phase. The detection of spot was carried out by using UV detector at 350 nm.The linearity of calibratin curve was found to be between 400-1600 ng per spot.
CONCLUSION:
A large number of techniques are available for theestimation ofnitazoxanide in pharmaceutical formulations andbiological samples. The survey of analytical data revealedthatHPLC methods are predominant for the estimation ofdrug alone or in combination with other drugs in variousformulation types. So for the precise and accurateseparation of nitazoxanidein various formulationsrecommended method of analysis includes HPLC with UVdetector as it provides faster analysis time and has moreseparation selectivity than most other available techniques.
This review carried out an overview of the state-of-artanalytical methods for the determination of nitazoxanidein different formulations using various analytical techniques.
REFERANCES:
1. Rossignol, J.F and Cavier, R. 1976. New derivative of 2-benzamido-5-nitrothiazoles. ChemAbstr. 83, 28.
2.Fox, L.M., and Saravolatz, L.D., Clinical Infectious Diseases, 40, 2005, 1173.
3.Raether, W. and Hanel, H. 2003. Nitroheterocyclic drugs with broad spectrum activity. Parasitol. 90, 19-39.
4.Cravier, R. 1978. Development and validation of spectrophotometric methods for the estimation of nitazoxanide in tablet dosage forms. Eur. J. Med. Chem. 13, 539.
5.O'Neil, M.J. 2001. An Encyclopedia of Chemicals, Drugs and Biologicals. The Merck Index. 13th edition, pp.1177-1178.
6.Murphy, J.R. and Friedmann, J.C. 1985. Pre-clinical toxicology of nitazoxanide, a new antiparasitic compound. J. Appl. Toxicol. 5, 49-52.
7.Stockis, A., Deroubaix, X., Lins, R., Jeanbaptiste, B., Calderon, P. and Rossignol, J.F. 1996. Pharmacokinetics ofnitazoxanide after single oral dose administration in 6 healthy volunteers. Int. J. Clin. Pharmacol. Ther. 34, 349-351.
8.Rossignol JF, Maisonneuve H (1984) Nitazoxanide in the treatment of Taeniasaginata and Hymenolepis nana. Am J Trop Med Hyg 33: 511– 512.
9. www.drugbank.com.
10.www.wikipedia.com.
11. Sanjay Sharma, Sachin Kumar.Nitazoxanide stabilizing hypsochromic shift based method for its determination in bulk and in pharmaceutical formulationMIT International Journal of Pharmaceutical Sciences, Vol. 1, No. 1, January 2015, pp. 13–18.
12. LopamudraAdhikari, SanjivSahu, SarbeswarJagdev. Development and Validation of Spectrophotometric and Colorimetric Method for the determination of Nitazoxanide in its Bulk and Pharmaceutical Dosage Form (Tablets).International Journal of ChemTech Research Vol.3, No.1, 131-135.
13.I. S. Sharma and M.C. Sharma.Development of Visible Spectrophotometric Methods for the Estimation of Nitazoxanide in Bulk and Pharmaceutical Formulation Using Ferric Chloride. American-Eurasian Journal of Scientific Research 6 (3): 155-160, 2011.
14.Fahima Aktar, Md. Ruhul Kuddus, Akter Hossen, Md. Khalid Hossain and Mohammad A. Rashid. Development and Validation of a Simple and Rapid UVSpectrophotometric Method for Assay of Nitazoxanide inPharmaceutical Dosage FormsBangladesh Pharmaceutical Journal 15(1): 47-51, 2012.
15.H.P.Singh, C. S. Sharma, Amar Deep Ankalgi, S. K. Agal and M. S. Ranawat. Spectrophotometric Methods for Simultaneous Determination of Nitazoxanideand Ofloxacin in Combined Bulk and Pharmaceutical FormulationsInternational Journal of PharmTech Research Vol. 3, No.1, 118-123.
16.Rajeev Jain, RajeevKumarYadav,JahangirAhmadRather. Voltammetric quantitation of nitazoxanide by glassy carbon electrode Journal ofPharmaceuticalAnalysis2013;3(6):452–455.
17.Hanaa S. El-Desoky; Mohamed M. Ghoneim; Mohamed M. Abdel-Galeil Stripping voltammetric methods for determination of the antiparasitic drug nitazoxanide in bulk form, pharmaceutical formulation and human serum,Journal of the Brazilian Chemical SocietyPrint version ISSN 0103-5053 J. Braz. Chem. Soc. 21:.4, 2010.
18.Hemlata Sharma, K K Jhankal, Ramlal Saini, D. K. Sharma,Voltammetric Assay of Antiviral Drug Nitazoxanide in Bulk Form, Human Breast Milk and Urine Sample International Journal of Recent Research and Review, Vol. III, September 2012.
19.Shital Gandhi, V. Mehta, and Sadhana Rajput, Simultaneous Voltammetric Determination of Nitazoxanide and Ofloxacin in Pharmaceutical Formulation Indian J Pharm Sci. 2011 Sep-Oct; 73(5): 583–586.
20.Ghada M. Hadad, Randa A. Abdel Salam and Samy Emara, Validated and Optimized High-Performance Liquid Chromatographic Determination of Tizoxanide, The Main Active Metabolite of Nitazoxanide in Human Urine, Plasmaand Breast Milk Journal of Chromatographic Science, April 23, 2012.
21.Maha Hegazy, Amira Kessiba, Ahmed Emad E Gindy and Mohamed Abdelkawy, ValidatedStability Indicating RP-HPLC for Quantitation of Nitazoxanidein Presence of Its Alkaline Degradation Products and Their Characterizationby HPLC-Tandem Mass Spectrometry journal ofChromatographic Science Journal of Chromatographic Science, November17, 2013;1–11.
22. H1. M.C. Sharma and S. SharmaDevelopment and Validation of a Dissolution method with IsocraticHigh-Performance Liquid Chromatographic Determination of tazoxanide and Ofloxacin in Pharmaceutical Dosage formAmerican-Eurasian Journal of Scientific Research 6 (4): 182-187, 2011
23. M. Sangeetha*1 and C. Mahendran2 RP-HPLC method for simultaneous estimation of Ofloxacin and Nitazoxanide in tablet formulationJournal of Chemical and Pharmaceutical Research, 2014, 6(9):365-369
24. . Hemendra P. Singh*, Sourabh K. Agal, Chandra S. Sharma, M S Ranawat and A D AnkalgiRP-HPLC methods for estimation of Nitazoxanide single and simultaneous estimation of Nitazoxanide with Ofloxacin in pharmaceutical dosage forms Journal of Chemical and Pharmaceutical ResearchJ. Chem. Pharm. Res., 2010, 2(4): 984-992
25. Jimish R Patel1*, Harpreet Singh Bedi2, Anil Middha2, Laxman M Prajapati1, Vijay K Parmar3RP-HPLC Method for Estimation of Nitazoxanide in Oral Suspension FormulationDer Pharma Chemica, 2012, 4 (3): 1140-1144(http://derpharmachemica.com/archive.html)
26. Selvadurai Muralidharan* Jaya raja Kumar and Sokkalingam Arumugam Dhanaraj Simultaneous Estimation of Nitazoxanide and Ofloxacin from Tablet Dosage Form by RP-HPLC Vol 1/Issue 4/July-August 2012 PhTechMed
27.T. Sakamoto, Y. Hiyama, Rapid determination of nitazoxanide in tablets using reversed-phase ultra-performance liquid chromatography (UPLC) and high-performance liquid chromatography, Pharmazie, 63: 2008, 503–507.
28.Jiang Shan , Zhou Dayong , Zhen WenhuaDetermination of Residual Solvents in Nitazoxanide by Headspace Gas Chromatography, Journal: China Pharmacist, Issue: 8, 2013, 1154 – 1156.
29. Zhanzhong Zhao, Lifang Zhang, Feiqun Xue , Xiaoyang Wang, Wenli Zheng, Tao Zhang, Chenzhong Fei, Keyu Zhang, Minqi Qiu, Ruixiang Xin, Fengkun Yang,Liquid chromatography–tandem mass spectrometry analysis of nitazoxanide and its major metabolites in goatJournal of Chromatography BVolume 875, Issue 2, 15 November 2008, 4.
30. Huang X, Guo C, Chen Z, Liu Y, He L, Zeng Z, Yan C, Pan G, Li S. Metabolism ofnitazoxanide in rats, pigs, and chickens: Application of liquid chromatography coupled to hybrid linear ion trap/Orbitrap mass spectrometer J Chromatogr B Analyt Technol Biomed Life Sci., 2015 Sep 1;1000:147-54.
31.M.D. Malesuik, H.M.L. Gonçalves, C.S. Paim, E.E.S. Schapoval, and M. Steppe, LC: Analysis of Photodegradation Kinetics of Nitazoxanide in Pharmaceutical Formulations Journal of Chromatographic Science, Vol. 47, October 2009
32.Salvador Namur, Lizbeth Carino, Mario Gonzalez-de la Parra,Development and validation of a high-performance thin-layer chromatographic method, with densitometry, for quantitative analysis of tizoxanide (a Metabolite of Nitazoxanide) in human plasma, November 08, 2007.
33.C L Gopu, Shibu Thomas, A R Paradkar and KR Mahadik , A validated stabilityindicating HPTLC method for determination of nitazoxanide. Journal of scientific and industrial research vol.66, February 2007, 141-145.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









