{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Devender Sharma1, Aashiya Aara E. Ali2, Jayshree R. Aate1
1Hi-Tech College of pharmacy, Chandrapur, Maharashtra (India)
2Kamla Nehru College of pharmacy, Nagpur (India)
*sdevender350@gmail.com
ABSTRACT
Niosome are non-ionic surfactant vesicles obtained by hydrating mixture of cholesterol and nonionic surfactants. It can be used as carriers of amphiphilic and lipophilic drug. In niosomes drug delivery system, the medication is encapsulated in a vesicle. Niosomes are biodegradable, biocompatible non-immunogenic and exhibit flexibility in their structural characterization. The main object of this review the application of niosome technology is used to treat a number of diseases, niosome have good oppurnity in research and beneficial for researcher and pharma industries. Niosome appears to be a well preferred drug delivery system over liposome as niosome being stable and economic. Also niosomes have great drug delivery potential for targeted delivery of anti-cancer, anti-infective agents. Drug delivery potential of niosome can enhances by using novel drug delivery concepts like proniosomes, discomes and aspasome. Niosomes also serve better aid in diagnostic imaging and as a vaccine adjuvant. Thus these areas need further exploration and research so as to bring out or to make for commercially available niosomal preparation.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2571
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 3 Received On: 04/01/2018; Accepted On: 04/01/2018; Published On: 01/03/2018 How to cite this article: Sharma D, Ali AAE, Aate JR; Niosomes as Novel Drug Delivery System: Review Article; PharmaTutor; 2018; 6(3); 58-65; http://dx.doi.org/10.29161/PT.v6.i3.2018.58 |
INTRODUCTION
Paul Ehrlich, in 1909, initiated the era of development for targeted delivery when he envisaged a drug delivery mechanism that would target directly to diseased cell. Drug targeting can be defined as the ability to direct a therapeutic agent specifically to desired site of action with little or no interaction with non target tissue. In niosomes drug delivery system the medication is encapsulated in a vesicle. (Baillie et al., 1985) The vesicle is composed of a bilayer of non-ionic surface active agents and hence the name niosomes. In niosomes, the vesicles forming amphiphilic is a non-ionic surfactant such as Span – 60 which is usually stabilized by addition of cholesterol and small amount of anionic surfactant such as dicetyl phosphate. (Hunter et al., 1988)
|
Advantages of niosomes |
Disadvantages of niosomes |
|
1. The characteristics such as size, lamellarity etc. of the vesicle can be varied depending on the requirement. |
1. Fusion |
|
2. The vesicles can act as a depot to release the drug slowly and offer a controlled release. |
2. Aggregation |
|
3. Since the structure of the niosome offers place to accommodate hydrophilic, lipophilic as well as amphiphilic drug moieties, they can be used for a variety of drugs. |
3. Leaking of entrapped drug |
|
4. The vesicle suspension being water based offers greater patient compliance over oil based systems |
4. Physical instability |
|
5. They are osmotically active and stable. |
5. Hydrolysis of encapsulated drugs which limiting the shelf life of the dispersion. |
|
6.They increase the stability of the entrapped drug |
|
|
7. Can enhance the skin penetration of drugs. |
|
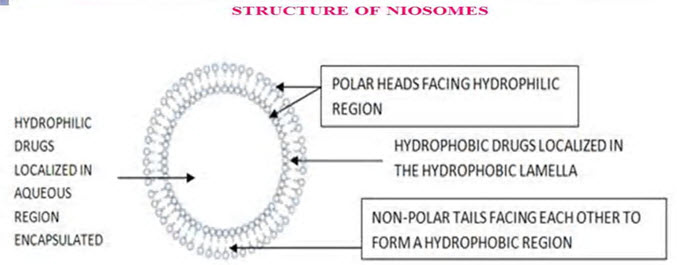
Structure of niosome (Gayatri et al., 2000)
A typical niosome vesicle would consist of a vesicle forming amphiphilic i.e. a non-ionic surfactant such as Span-60, which is usually stabilized by the addition of cholesterol and a small amount of anionic surfactant such as dicetyl phosphate, which also helps in stabilizing the vesicle.
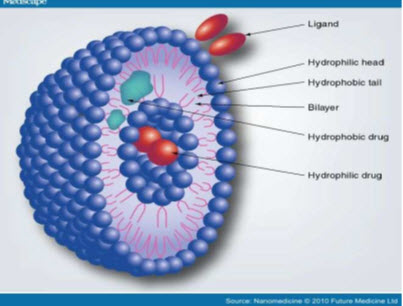
Compositions of niosomes: (Gayatri et al., 2000)
The two major components used for the preparation of niosomes are,
1. Cholesterol
2. Nonionic surfactants
1. Cholesterol
Cholesterol is a steroid derivative, which is used to provide rigidity and proper shape, conformation to the niosomes preparations.
2. Nonionic surfactants
The following non-ionic surfactants are generally used for the preparation of niosomes. e.g.
1. Spans (span 60, 40, 20, 85, 80)
2. Tweens (tween 20, 40, 60, 80)
3. Brijs (brij 30, 35, 52, 58, 72, 76)
The non ionic surfactants possess a hydrophilic head and a hydrophobic tail.
Preparation methods of niosomes
Ether injection method
The ether injection method is essentially based on slowly introducing a solution of surfactant dissolved in diethyl ether into warm water maintained at 60°C. The surfactant mixture in ether is injected through 14-gauge needle into an aqueous solution of material. Vaporization of ether leads to formation of single layered vesicles. The particle size of the niosomes formed depend on the conditions used the diameter of the vesicle range from 50 to 1000 nm.
Hand shaking method (thin film hydration technique): (Khandare et al., 1994)
In this method the surfactant and cholesterol are dissolved in a volatile organic solvent (such as diethyl either, chloroform or methanol) in a round bottom flask. The organic solvent is removed at room temperature (20°C) using rotary evaporator leaving a thin layer of solid mixture deposited on the wall of the flask. The dried surfactant film can be rehydrated with aqueous phase at 0-60°C with gentle agitation to yield multilamellar niosomes.
Sonication Method
In this method an aliquot of drug solution in buffer is added to the surfactant/cholesterol mixture in a 10-ml glass vial. The mixture is probe sonicated at 60°C for 3 minutes using a sonicator with a titanium probe to yield niosomes.
Micro fluidization method (Rogerson et al., 1988)
Micro fluidization is a recent technique used to prepare unilamellar vesicles of defined size distribution. This method is based on submerged jet principle in which two fluidized streams interact at ultra high velocities, in precisely defined micro channels within the interaction chamber. The impingement of thin liquid sheet along a common front is arranged such that the energy supplied to the system remains within the area of niosomes formation. The result is a greater uniformity, smaller size and better reproducibility of niosomes formed.
Multiple membrane extrusion method
Mixture of surfactant, cholesterol and dicetyl phosphate in chloroform is made into thin film by evaporation. The film is hydrated with aqueous drug polycarbonate membranes, solution and the resultant suspension extruded through which are placed in series for upto 8 passages. It is a good method for controlling noisome size.
Reverse Phase Evaporation Technique (REV) (Biju et al., 2006)
In this method, Cholesterol and surfactant (1:1) are dissolved in a mixture of ether and chloroform. An aqueous phase containing drug is added to this and the resulting two phases are sonicated at 4-5°C. A clear gel is formed which is further sonicated after the addition of phosphate buffered saline (PBS). The organic phase is removed at 40°C under low pressure. The resulting viscous noisome suspension is diluted with PBS and heated on a water bath at 60°C for 10 min to yield niosomes.
Transmembranes pH gradient (inside acidic) Drug Uptake Process: or Remote Loading Technique (Biju et al., 2006)
A solution of surfactant and cholesterol are dissolved in chloroform. The solvent is then evaporated under reduced pressure to get a thin film on the wall of the round bottom flask. This film is hydrated with 300mm citric acid (PH 4.00) by vertex mixing. The resulting multilamellar vesicles are frozen and shared three times and later sonicated. To this niosomal suspension. Aqueous solution containing 10 mg/ml of drug is added and vortexes. The PH of the sample is then raised to 7.0-7.2 with 1M disodium phosphate. This mixture is later heated at 60°c for 10 minutes to give niosomes.
The Bubble Method (Ijeoma et al., 1998)
The bubbling unit consists of round-bottomed flask with three necks, and this is positioned in a water bath to control the temperature. Water-cooled reflux and thermometer is positioned in the first and second neck and nitrogen supply through the third neck. Cholesterol and surfactant are dispersed together in this buffer (PH 7.4) at 70°C, the dispersion mixed for 15 seconds with high shear homogenizer and immediately afterwards “bubbled” at 70°C using nitrogen gas to yield niosomes. (Breimer et al., 1985)
Factors affecting niosomes formulation (Handjani et al., 1979)
Drug
Entrapment of drug in niosomes influence charge and rigidity of the noisome bilayer. The hydrophilic lipophilic balance of the drug affects degree of entrapment.
Nature and type of surfactant (Malhotra et al., 1994)
The mean size of niosomes increases proportionally with increase in the HLB surfactants like Span 85 (HLB 1.8) to Span 20 HLB 8.6) because the surface free energy decreases with an increase in hydrophobicity of surfactant. A surfactant must have a hydrophilic head and hydrophobic tail. The hydrophobic tail may consist of one or two alkyl or perfluoroalkyl groups or in some cases a single steroidal group.
Cholesterol content and charge (Weissman et al., 1975) (Alsarra et al., 2004)
Hydrodynamic diameter and entrapment efficiency of niosomes is increased by cholesterol. It induces membrane stabilizing activity and decreases the leakiness of membrane. An increase in cholesterol content of the bilayers resulted in a decrease in the release rate of encapsulated material and therefore an increase of the rigidity of the bilayers obtained. Presence of charge tends to increase the interlamellar distance between successive bilayers in multilamellar vesicle structure and leads to greater overall entrapped volume.
Resistance to osmotic stress
The diameter is reduced by addition of hypertonic salt solution to suspension of niosomes.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Temperature of Hydration
Hydration temperature influences the shape and size of niosome.
Characterization of niosomes (Hu et al., 1994)
Measurement of Angle of repose
The angle of repose of dry niosomes powder was measured by a funnel method. The niosomes powder was poured into a funnel which was fixed at a position so that the 13mm outlet orifice of the funnel is 5cm above a level black surface. The powder flows down from the funnel to form a cone on the surface and the angle of repose was then calculated by measuring the height of the cone and the diameter of its base.
Scanning electron microscopy (Sternberg., 1998)
Particle size of niosomes is very important characteristic. The surface morphology (roundness, smoothness, and formation aggregates) and the size distribution of niosomes were studied by Scanning Electron Microscopy (SEM). Niosomes were sprinkled on to the double- sided tape that was affixed on aluminum stubs. The aluminum stub was placed in the vacuum chamber of a scanning electron microscope (XL 30 ESEM with EDAX, Philips, Netherlands). The samples were observed for morphological characterization using a gaseous secondary electron detector (working pressure: 0.8 torr, acceleration voltage: 30.00 KV) XL 30, (Philips, Netherlands).
Optical Microscopy
The niosomes were mounted on glass slides and viewed under a microscope (Medilux-207RII, Kyowa-Getner, Ambala, India) with a magnification of 1200X for morphological observation after suitable dilution. The photomicrograph of the preparation also obtained from the microscope by using a digital SLR camera.
Measurement of vesicle size (Sternberg., 1998)
The vesicle dispersions were diluted about 100 times in the same medium used for their preparation. Vesicle size was measured on a particle size analyzer (Laser diffraction particle size analyzer, Sympatec, Germany). The apparatus consists of a He-Ne laser beam of 632.8 nm focused with a minimum power of 5 mW using a Fourier lens [R-5] to a point at the center of multielement detector and a small volume sample holding cell (Su cell). The sample was stirred using a stirrer before determining the vesicle size. Hu C. and Rhodes 7 in 1999 reported that the average particle size of niosomes derived niosomes is approximately 6µm while that of conventional niosomes is about 14µm.
Entrapment efficiency (Sternberg., 1998)
Entrapment efficiency of the niosomal dispersion in can be done by separating the unentrapped drug by dialysis centrifugation or gel filtration as described above and the drug remained entrapped in niosomes is determined by complete vesicle disruption using 50% n-propanol or 0.1% Triton X-100 and analyzing the resultant solution by appropriate assay method for the drug. Where,
Osmotic shock (Sternberg., 1998)
The change in the vesicle size can be determined by osmotic studies. Niosomes formulations are incubated with hypotonic, isotonic, hypertonic solutions for 3 hours. Then thechanges in the size of vesicles in the formulations are viewed under optical microscopy.
Stability studies(Theresa., 1988)
To determine the stability of niosomes, the optimizedbatch was stored in airtight sealed vials at different temperatures. Surface characteristics and percentage drug retained in niosomes and niosomes derived from proniosomes were selected as parameters for evaluation of the stability, since instability of the formulation would reflect in drug leakage and a decrease. In the percentage drug retained. The niosomes were sample at regular intervals of time (0,1,2,and 3months ),observed for color change, surface characteristics and tested for the percentage drug retained after being hydrated to form niosomes and analyzed by suitable analytical methods(UV spectroscopy, HPLC methods etc).
Zeta potential analysis (Buckton et al., 1995)
Zeta potential analysis is done for determining the colloidal properties of the prepared formulations. The suitably diluted niosomes derived from pronoisome dispersion was determined using zeta potential analyzer based on electrophoretic light scattering and laser Doppler velocimetry method (Zeta plus™, Brookhaven Instrument Corporation, New York, USA). The temperature was set at 25°C. Charge on vesicles and their mean zeta potential values with standard deviation of measurements were obtained directly from the measurement.
In-vitro methods for niosomes (Reddy et al., 1993)
In vitro drug release can be done by
1. Dialysis tubing
2. Reverse dialysis
3. Franz diffusion cell
Dialysis tubing
Muller et al, in 2002 studied in vitro drug release could be achieved by using dialysis tubing. The niosomes is placed in prewashed dialysis tubing which can be hermetically sealed. The dialysis sac is then dialyzed against a suitable dissolution medium at room temperature; the samples are withdrawn from the medium at suitable intervals, centrifuged and analyzed for drug content using suitable method (U.V. spectroscopy, HPLC etc). The maintenance of sink condition is essential. (Shahiwala et al., 2002)
Reverse dialysis (Satturwar., 2002)
In this technique a number of small dialysis as containing 1ml of dissolution medium are placed in proniosomes. The proniosomes are then displaced into the dissolution medium. The direct dilution of the proniosomes is possible with this method; however the rapid release cannot be quantified using this method. (Azmin et al., 2005)
Franz diffusion cell
The in vitro diffusion studies can be performed by using Franz diffusion cell. Proniosomes is placed in the donor chamber of a Franz diffusion cell fitted with a cellophane membrane. The proniosomes is then dialyzed against a suitable dissolution medium at room temperature; the samples are withdrawn from the medium at suitable intervals, and analyzed for drug content using suitable method (U.V spectroscopy, HPLC, etc) .the maintenance of sink condition is essential. (Weissman et al., 1975) (Alsarra et al., 2004)
|
Evaluation parameter |
Generally used method in evaluation parameter |
|
Morphology |
SEM, TEM, freeze fracture technique |
|
Size distribution, polydispersity index |
Dynamic light scattering particle size analyzer |
|
Viscosity |
Ostwald viscometer |
|
Membrane thickness |
X-ray scattering analysis |
|
Thermal analysis |
DSC |
|
Turbidity |
UV-Visible diode array spectrophotometer |
|
Entrapment efficacy |
Centrifugation, dialysis, gel chromatography |
|
In-vitro release study |
Dialysis membrane |
|
Permeation study |
Franz diffusion cell |
Applications of niosomes (Weissman et al., 1975) (Alsarra et al., 2004)
1. Niosomes have been used for studying the nature of the immune response provoked by antigens.
2. It is used as Drug Targeting.
3. It is used as Anti-neoplastic Treatment i.e. Cancer Disease.
4. It is used as Leishmaniasis i.e. Dermal and Mucocutaneous infections e.g. Sodium stibogluconate.
5. Niosomes as Carriers for Hemoglobin.
6. It is used act as Delivery of Peptide Drugs.
7. Niosomes can be used as a carrier for hemoglobin.
8. It is used in Studying Immune Response.
9. Transdermal Drug Delivery Systems Utilizing Niosomes.
10. It is used in ophthalmic drug delivery.
11. Niosomal system can be used as diagnostic agents.
Immunological application of niosomes (Navneet et al., 2014)
Niosomes have been used for studying the nature of the immune response provoked by antigens. Niosomes can also be utilized for targeting drugs to organs other than the Reticulo-Endothelial System. A carrier system (such as antibodies) can be attached to niosomes (as immunoglobulin’s bind readily to the lipid surface of the niosome) to target them to specific organs.
Sustained Release (Navneet et al., 2014)
Sustained release action of niosomes can be applied to drugs which have low therapeutic index and have low solubility with water since those could be maintained in the circulation via niosomal encapsulation.
Localized Drug Action (Navneet et al., 2014)
Drug delivery through niosomes is one of the approaches to achieve localized drug action, since their size and low penetrability through epithelium and connective tissue keeps the drug localized at the site of administration
Niosomes as Drug Carriers (Navneet et al., 2014)
Niosomes have also been used as carriers for iobitridol, a diagnostic agent used for X-ray imaging. Topical niosomes may serve as solubilization matrix, as a local depot for sustained release of dermally active compounds, as penetration enhancers, or as rate-limiting membrane barrier for the modulation of systemic absorption of drugs.
Transdermal delivery of drugs by niosomes (Navneet et al., 2014)
Those drug have slow penetration of medicament through skin is the major drawback of transdermal route of delivery. An increase in the penetration rate has been achieved by transdermal delivery of drug incorporated in niosomes. From the above discussed studies, and confocal microscopy, it was seen that non-ionic vesicles could be formulated to target pilosebaceous glands. Topical niosomes may serve as solubilization matrix, as a local depot for sustained release of dermally active compounds, as penetration enhancers, or as rate-limiting membrane barrier for the modulation of systemic absorption of drugs.
Leishmaniasis (Navneet et al., 2014)
Leishmaniasis is a disease in which a parasite of the genus Leishmania invades the cells of the liver and spleen. Use of niosomes in tests conducted showed that it was possible to administer higher levels of the drug without the triggering of the side effects, and thus allowed greater efficacy in treatment.
Delivery of Peptide Drugs (Navneet et al., 2014)
Oral peptide drug delivery has long been faced with a challenge of bypassing the enzymes which would breakdown the peptide. Use of niosomes to successfully protect the peptides from gastrointestinal peptide breakdown is being investigated. In an in vitro study conducted by oral delivery of a vasopressin derivative entrapped in niosomes showed that entrapment of the drug significantly increased the stability of the peptide.
Niosome formulation as a brain targeted delivery system for the vasoactive intestinal peptide
Radiolabelled (I125) VIP-loaded glucosebearing niosomes were injected intravenously to mice. Encapsulated VIP within glucosebearing niosomes exhibits higher VIP brain uptake as compared to control.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Niosomes as carriers for Hemoglobin (Navneet et al., 2014)
Niosomes can be used as a carrier for hemoglobin. Niosomal suspension shows a visible spectrum superimposable which is likely to be or, onto that of free hemoglobin. Vesicles are permeable to oxygen and hemoglobin dissociation curve can be modified similarly to non-encapsulated hemoglobin. Anti-neoplastic Treatment Most antineoplastic drugs cause severe side effects. Niosomes can alter the metabolism; prolong circulation and half life of the drug, thus decreasing the side effects of the drugs. Niosomes are decreased rate of proliferation of tumor and higher plasma levels accompanied by slower elimination.
Drug Targeting (Navneet et al., 2014)
One of the most useful aspects of niosomes is their ability to target drugs. Niosomes can be used to target drugs to the reticuloendothelial system. The reticulo-endothelial system (RES) preferentially takes up niosome vesicles. The uptake of niosomes is controlled by circulating serum factors called opsonins. These opsonins mark the niosome for clearance. Such localization of drugs is utilized to treat tumors in animals known to metastasize to the liver and spleen. This localization of drugs can also be used for treating parasitic infections of the liver. Niosomes can also be utilized for targeting drugs to organs other than the RES. A carrier system (such as antibodies) can be attached to niosomes (as immunoglobulin’s bind readily to the lipid surface of the niosome) to target them to specific organs.
Route of application of niosomes drugs (Gayatri et al., 2000)
|
Route of administration |
Examples of drug |
|
Intravenous route |
Doxorubicin, comptothecin, insulin, zidovudine, cisplatin, rifampicin |
|
Inhalation |
All trans-retonic acids |
|
Transdermal route |
Piroxicam, estradiol, nimesulide |
|
Ocular route |
Timolol maleate,cyclopentol |
|
Nasal route |
Sumatriptan, influenzaviral vaccines |
CONCLUSION
Niosomal drug delivery system is one of the best examples of great evolution in drug delivery technologies and nanotechnology. It is obvious that niosome appears to be a well preferred drug delivery system over other dosage form as niosome mostly stable in nature and economic. There is lot of scope to encapsulate toxic anti-cancer drugs, anti-infective drugs, anti-AIDS drugs, anti-inflammatory drugs, anti-viral drugs, etc. in niosomes and to use them as promising drug carriers to achieve better bioavailability and targeting properties and for reducing the toxicity and side effects of the drugs. Thus these areas require further systemic consideration and research so as to bring out commercially and valuable available niosomal preparation. The concept of incorporating the drug into or niosomes for a better targeting of the drug at appropriate tissue destination is widely accepted by researchers and academicians. The ionic drug carriers are relatively toxic and unsuitable whereas niosomal carriers are safer. And also handling and storage of niosomes require no special conditions. Niosomes represent a promising drug delivery module. They have similar structure to liposome, to little same in property and hence they can represent alternative vesicular systems with respect to liposomes, due to the niosome ability to encapsulate different type of drugs within their multi-environmental structure. Niosomes are thoughts to be better candidate drug delivery as compared to liposomes due to various factors like cost, stability etc. Niosomes have very important and key role in various types of drug deliveries; like targeting, topical, ophthalmic and parenteral. Niosomes are very useful in bright future for pharma industries. So far only animal experimentation of this targeted drug delivery system is reported but further clinical investigations in human volunteers, pharmacological and toxicological investigations in animals and human volunteers may help to exploit niosomes as prosperous drug carriers for targeting drugs more efficiently, for treating cancer, infection and AIDS etc.
REFERENCES
1. Alsarra A., Bosela A,, Ahmed S.M,, Mahrous G.M., Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur. J. Pharm. And Biopharm. 2004; 2(1): 1-6.
2. Azmin MN, Florence AT, Handjani-Vila RM, Stuart JF, Vanlerberghe G, Whittaker JS. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J Pharm Pharmacol 2005;37:237-42.
3. Baillie AJ., Florence AT., Hume LR., Muirhead GT., Rogerson A., The preparation and properties of niosomes non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1985, 37: 863- 868.
4. Biju SS., Talegaonkar S., Misra PR., Khar RK., Vesicular systems: An overview. Indian J. Pharm. Sci. 2006, 68: 141-153.
5. Breimer DD and Speiser R. Topics in Pharmaceutical Sciences. Elsevier Science Publishers, New York, USA. 1985;291.
6. Buckton G and Harwood. Interfacial Phenomena in Drug Delivery and Targeting Academic Publishers, Switzerland. 1995;154-155.
7. Gayatri Devi S, Venkatesh P, Udupa N. Niosomal sumatriptan succinate for nasal administration. Int J Pharm Sci 2000;62:479-81.
8. Handjani VRM. Dispersion of Lamellar Phases of Nonionic Lipids in Cosmetic Products. Int J Cosmetic Sc. 1979;30.
9. http//en.wikipedia.org/wiki/Niosomes, structure of niosomes.
10. Hu C., Rhodes D.G., Proniosomes: a novel drug carrier preparation. Int. J. Pharm. 1999, 185: 23-35.
11. Hunter CA, Dolan TF, Coombs G, Baillie AJ. Vesicular systems (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J Pharm Pharmacol 1988; 40:161-5.
12. Ijeoma F., Uchegbu., Suresh P., Vyas., Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998; 172: 33–70.
13. Khandare JN., Madhavi G., Tamhankar BM., Niosomes Novel Drug Delivery System. The Eastern Pharmacist. 1994, 37: 61-64.
14. Malhotra M., Jain N.K., Niosomes as Drug Carriers. Indian Drugs. 1994, 31(3): 81-866.
15. Navneet Kumar Verma, Asha Roshan. Niosomes and its application:A Review, IJRPLS,2014. 2(1): 182-184.
16. Reddy DN and Udupa N. Formulation and Evaluation of Oral and Transdermal Preparation of Flurbiprofen and Piroxicam Incorporated with Different Carriers. Drug Dev Ind Pharm. 1993;843.
17. Rogerson A., Cummings J., Willmott N., Florence AT., The distribution of doxorubicin in mice following administration in niosomes. J. Pharm. Pharmacol. 1988, 40: 337-342.
18. Satturwar P M. Formulation and Evaluation of Ketoconazole Niosomes. Ind J Pharm Sci. 2002;155.
19. Shahiwala A and Misra A. Studies in Topical Application of Niosomally Entrapped Nimesulide. J Pharma Sci. 2002;220.
20. Sternberg B, Uchegbu IF, Florence AT and Murdan S. 1998.
21. Theresa MA. Drugs published by ADIS international Ltd. 1998;56(5):747-756.
22. Weissman G, Bloomgarden D, Kaplan R, Cohen C, Hoffstein S, Collins T, et al. A general method for the introduction of enzymes, by means of immunoglobulin-coated liposomes, into lysosomes of deficient cells. Proc Natl Acad Sci 1975;72:88-92.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









