 About Authors:
About Authors:
Ashish Kumar Garg*, M.M. Gupta
Department of Pharmaceutics, Jaipur College of Pharmacy,
Jaipur, Rajasthan
*ashish1pharma@gmail.com
Abstract
Conventional dosage forms like tablets and capsules are now days facing the problems like dysphagia, resulting in the high incidence of non compliance and making the therapy ineffective. To obviate the problems associated with conventional dosage forms, mouth dissolving tablets have been developed having good hardness, dose uniformity, easy administration and serves as the first choice of dosage form for paediatrics, geriatrics and traveling patients. The MDTs were developed with an aim of having sufficient hardness, integrity and faster disintegration without water. Fast dissolving Tablets are disintegrating and/or dissolve rapidly in the saliva without the need for water. Some tablets are designed to dissolve in saliva remarkably fast, within a few seconds, and are true fast-dissolving tablets. Others contain agents to enhance the rate of tablet disintegration in the oral cavity, and are more appropriately termed fast-disintegrating tablets, as they may take up to a minute to completely disintegrate. This tablet format is designed to allow administration of an oral solid dose form in the absence of water or fluid intake. Such tablets readily dissolve or disintegrate in the saliva generally within <60 seconds.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1567
INTRODUCTION
Despite of tremendous innovations in drug delivery, the oral route remains the preferred route for administration of therapeutic agents because of accurate dosage, low cost therapy, self medication, non invasive method and ease of administration leading to high level of patient compliance[1]. However, traditional tablets and capsules administered with a glass of water may be inconvenient or impractical for some geriatric patients because of changes in various physiological and neurological conditions associated with aging including difficulty in swallowing/dysphagia, hand tremors, deterioration in their eyesight, hearing, memory, risk of choking in addition to change in taste and smell. Solid dosage forms also present significant administration challenges in other patient groups, such as children, mentally challenged, bed ridden and uncooperative patients. Pediatric patients may suffer from ingestion problems as a result of underdeveloped muscular and nervous control. Moreover, patients traveling with little or no access to water, limit utility of orally administered conventional tablets or capsules.
Therefore, to cater the needs of such patients, recent advancements in technology have resulted in development of viable dosage alternatives popularly known as orally disintegrating tablets (ODTs)[1,2].During the past decade, the FDT (fast dissolving tablet) technology, which makes tablets dissolve or disintegrate in the mouth without additional water intake, has drawn a great deal of attention.The technology is also referred to as fast disintegrating tablet, fast dispersing tablet, rapid dissolve tablet, rapid melt tablet, quick disintegrating tablet, and orally disintegrating tablet. The FDT formulation is defined by the Food and Drug Administration (FDA) as ‘‘a solid dosage form containing medicinal substances which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue’’. The tablets disintegrate into smaller granules or melt inthe mouth from a hard solid structure to a gel like structure, allowing easy swallowing by the patients. The disintegration time for those tablets varies from a few seconds to more than a minute[2,3].
FDT is a desirable dosage form for patients with problems swallowing tablets or other solid dosage forms. It has advantages over oral solutions including better stability, more accurate dosing, and lower volume and weight. The dosage form can be swallowed as a soft paste or liquid, and suffocation is avoided because there is no physical obstruction when swallowed. Since the tablets disintegrate in the mouth, drugs can be absorbed in the buccal, pharyngeal, and gastric regions. Thus, rapid drug therapy intervention and increased bioavailability of drugs might be possible.Because pre-gastric drug absorption avoids first pass metabolism, the drug dose can be reduced if a significant amount of the drug is lost through the hepatic metabolism.
Administration of FDTs is different from conventional tablets, and the FDTs should have several unique properties to accommodate the rapid disintegration time. They should dissolve or disintegrate in the mouth without water or with a very small amount of water as the disintegration fluid is the patient’s saliva. The disintegrated tablet should become a soft paste or liquid suspension, which provides good mouth feel and enables smooth swallowing. ‘‘Fast dissolution’’ or ‘‘fast disintegration’’ typically requires dissolution or disintegration of a tablet withinoneminute.[1,2]
Significance of ODTs [4,5]
ODTs offer dual advantages of solid dosage forms and liquid dosage forms along with special
Features which include:
· Accurate dosing: Being unit solid dosage forms, provide luxury of accurate dosing, easy portability and manufacturing, good physical and chemical stability and an ideal alternative for pediatric and geriatric patients.
· Enhanced bioavailability: Bioavailability of drugs is enhanced due to absorption from mouth , pharynx and esophagus.
· Rapid action: Fast onset of therapeutic action as tablet gets disintegrated rapidly along with quick dissolution and absorption in oral cavity.
· Patient compliance: No need of water to swallow the dosage form. Hence, it is convenient for patients who are traveling and do not have immediate access to water.
· Ease of administration: Convenient to administer specially for geriatric, pediatric, mentally disabled and bed ridden patients who have difficulty in swallowing.
· Obstruction free: No risk of suffocation in airways due to physical obstruction when swallowed, thus providing improved safety and compliance.
· Enhanced palatability: Good mouth feels, especially for pediatric patients as taste masking technique is used to avoid the bitter taste of drug.
· Simple packaging: No specific packaging required. It can be packaged in push through blisters.
· Business Avenue: Provide new business opportunities in the form of product differentiation, line extension, uniqueness and life cycle management.
· Cost effective: Conventional processing and packaging equipments allow the manufacturing of tablets at low cost.
Ideal Properties of MDTs[4]
They should –
* Not require water to swallow, but it should dissolve or disintegrate in the mouth in matter of seconds.
* Be compatible with taste masking.
* Be portable without fragility concern.
* Have a pleasant mouth feel.
* Leave minimum or no residue in the mouth after oral administration.
* Exhibit low sensitive to environmental condition as temperature and humidity.
* Allow the manufacture of the tablet using conventional processing and packaging equipments at low cost.
Challenges in formulating Fast dissolving tablets:[5,19]
Palatability
As most drugs are unpalatable, FDTs usually contain the medicament in a taste-masked form. Upon administration, it disintegrate or dissolve in patient’s oral cavity, thus releasing the active ingredients which come in contact with the taste buds. Hence, taste-masking of the drugs becomes critical to patient compliance .
Mechanical strength
In order to allow FDTs to disintegrate in the oral cavity, they are made of either very porous and soft-molded matrices or compressed into tablets with very low compression force, which makes the tablets friable and/or brittle, difficult to handle, andoften requiring specialized peel-off blister packing that may add to the cost. Only Wow tab and durasolv technologies can produce tablets that are sufficiently hard and durable to allow them to be packaged in multi-dose bottles.
Hygroscopicity
Several orally disintegrating dosage forms are hygroscopic and cannot maintain physical integrity under normal conditions of temperature and humidity. Hence, they need protection from humidity which calls for specialized product packaging.
Amount of drug
The application of technologies used for FDTs is limited by the amount of drug that can be incorporated into each unit dose. For lyophilized dosage forms, the drug dose must be less than 400 mg for insoluble drugs and 60 mg for soluble drugs. This parameter is particularly challenging when formulating a fast-dissolving oral films or wafers.
Aqueous solubility
Water-soluble drugs pose various formulation challenges because they form eutectic mixtures, which result in freezing-point depression and the formation of a glassy solid that may collapse upon drying because of loss of supporting structure during the sublimation process. Such collapse sometimes can be prevented by using various matrix-forming excipients such as mannitol that can induce crystallinity and hence, impart rigidity to the amorphous composite.
Size of tablet
The ease of administration of a tablet depends on its size. It has been reported that the easiest size of tablet to swallow is 7-8 mm while the easiest size to handle was one larger than 8 mm. Therefore, the tablet size that is both easy to take and easy to handle is difficult to achieve.
FORMULATION OF MDTs:[5,6,7,8]
Drug:
The ultimate characteristics of a drug for dissolution in the mouth and pre gastric absorption from MDTs include:
* Free from bitter taste
* Dose lower than 20 mg
* Small to Moderate molecular weight
* Good solubility in saliva
* Ability to permeate through oral mucosal tissue
Bulking materials:
Bulking materials are significant in the formulation of fast-melting tablets. The material contributes functions of a diluent, filler and cost reducer. Bulking agents improve the textural characteristics that in turn enhance the disintegration in the mouth, besides; adding bulk also reduces the concentration of the active in the composition. The recommended bulking agents for this delivery system should be more sugar-based such as mannitol, polydextrose, lactitol, DCL (direct compressible lactose) and starch hydrolystate for higher aqueous solubility and good sensory perception. Mannitol in particular has high aqueous solubility and good sensory perception. Bulking agents are added in the range of 10 percent to about 90 percent by weight of the final composition.
Emulsifying agents:
Emulsifying agents are important excipients for formulating fast-melting tablets they aid in rapid disintegration and drug release without chewing, swallowing or drinking water. In addition, incorporating emulsifying agents is useful in stabilizing the immiscible blends and enhancing bioavailability. A wide range of emulsifiers is recommended for fast-tablet formulation, including alkyl sulfates, propylene glycol esters, lecithin, sucrose esters and others. These agents can be incorporated in the range of 0.05 percent to about 15 percent by weight of the final composition.
Lubricants:
Lubricants, though not essential excipients, can further assist in making these tablets more palatable after they disintegrate in the mouth. Lubricants remove grittiness and assist in the drug transport mechanism from the mouth down into the stomach.
Flavours and sweeteners:
Flavours and taste-masking agents make the products more palatable and pleasing for patients. The addition of these ingredients assists in overcoming bitterness and undesirable tastes of some active ingredients. Both natural and synthetic flavours can be used to improve the organoleptic characteristic of fast-melting tablets. Formulators can choose from a wide range of sweeteners including sugar, dextrose and fructose, as well as non-nutritive sweeteners such as aspartame, sodium saccharin, sugar alcohols and sucralose. The addition of sweeteners contributes a pleasant taste as well as bulk to the composition.
Superdisintegrants:
A disintegrant is an excipient, which is added to a tablet or capsule blend to aid in the breakup of the compacted mass when it is put into a fluid environment.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table: 1 Enlists various existing superdisintegrants and also their mechanism of action.
|
Name of disintegrant |
Brand name |
Concentration(%) |
Mechanism of action |
|
Sodium Starch Glycolate
|
Explotab,Primogel |
2-8% |
Swelling |
|
Micro crystalline cellulose |
Avicel, Celex |
2-15% |
Water wicking |
|
Cross linked povidone |
Cross povidone |
2-5% |
Swelling, Water wicking |
|
Low substuted hydroxy propyl cellulose |
LH-11, LH-12 (Grades) |
1-5% |
Sweling |
|
Crosscarmellose sodium |
Ac-Di-Sol |
1-3%Direct compression 2-4% wet granulation |
Wicking and swelling |
|
Pregelatinized starch |
Starch 1500 |
1-20% |
Swelling |
Advantages:
1. Effective in lower concentrations.
2. Less effect on compressibility and flowabilit .
SELECTION OF SUPERDISINTEGRANTS:
Although superdisintegrants primarily affect the rate of disintegration, but when used at high levels they can also affect mouth feel, tablet hardness and friability. Hence, various ideal factors to be considered while selecting an appropriate superdisintegrants for a particular formulationshould:
• Produce rapid disintegration, when tablet comes in contact with saliva in the mouth/oral cavity.
• Be compactable enough to produce less friable tablets.
• Produce good mouth feel to the patients. Thus, small particle size is preferred to achieve patient compliance.
• Have good flow, since it improves the flow characteristics of total blend.
MECHANISM OF ACTION:
There are four major mechanisms for tablets disintegration as follows
1. Swelling:
Perhaps the most widely accepted general mechanism of action for tablet disintegration is swelling. Swelling is believed to be a mechanism in which certain disintegrating agents (such as starch) impart the disintegrating effect. By swelling in contact with water, the adhesiveness of other ingredients in a tablet is overcome causing the tablet to fall apart(Figure 1). Tablets with high porosity show poor disintegration due to lack of adequate swelling force. On the other hand, sufficient swelling force is exerted in the tablet with low porosity. It is worthwhile to note that if the packing fraction is very high, fluid is unable to penetrate in the tablet and disintegration is again slows down.
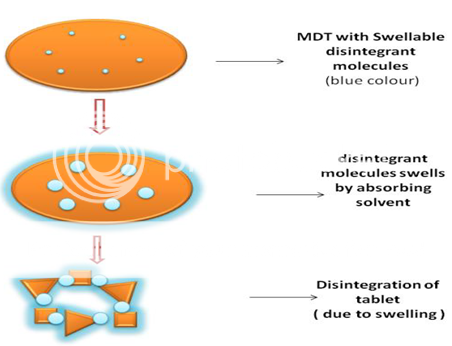
Figure 1: Swelling Mechanism of a Disintegrant in which the disintegrant swells, absorbs the surrounding medium aiding in fast disintegration
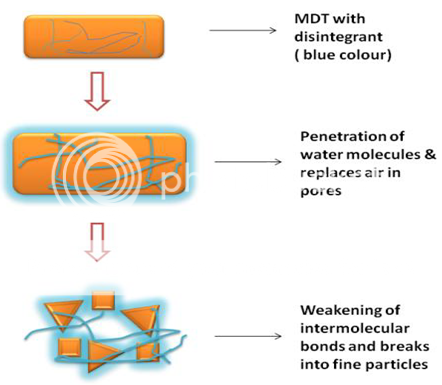
Figure 2: Wicking mechanism. This mechanism involves capillary action aiding faster disintegration
2. Porosity and capillary action (Wicking):
Effective disintegrants that do not swell are believed to impart their disintegrating action through porosity and capillary action. When we put the tablet into suitable aqueous medium, the medium penetrates into the tablet and replaces the air adsorbed on the particles, which weakens the intermolecular bond and breaks the tablet into fine particles. Tablet porosity provides pathways for the penetration of fluid into tablets. The disintegrant particles (with low cohesiveness &compressibility) themselves act to enhance porosity and provide these pathways into the tablet (Figure 2).Water uptake by tablet depends upon hydrophilicity of the drug /excipient and on tableting conditions. For these types of disintegrants maintenance of porous structure and low interfacial tension towards aqueous fluid is necessary which helps in disintegration by creating a hydrophilic network around the drug particles.
3. Due to disintegrating particle/particle repulsive forces:
Another mechanism of disintegration attempts to explain the swelling of tablet made with „non-swellable? disintegrants. Guyot-Hermann has proposed a particle repulsion theory based on the observation that non-swelling particle also cause disintegration of tablets (Figure 3). The electric repulsive forces between particles are the mechanism of disintegration and water is required for it. Researchers found that repulsion is secondary to wicking.
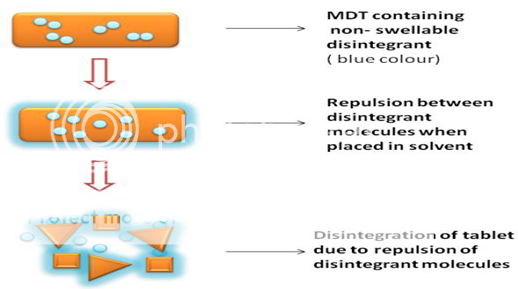
Figure 3: Repulsion mechanism of Disintegrant. In this mechanism, repulsion forces between the molecules causes tablet to disintegrate
4. Due to deformation:
During tablet compression, disintegrated particles get deformed and these deformed particles get into their normal structure when they come in contact with aqueous media or water. Occasionally, the swelling capacity of starch was improved when granules were extensively deformed during compression. This increase in size of the deformed particles produces a breakup of the tablet (Figure 4). Starch grains are generally thought to be “elastic” in nature meaning that grains that are deformed under pressure will return to their original shape when that pressure is removed. But, with the compression forces involved in tableting, these grains are believed to be deformed more permanently and are said to be “energy rich” with this energy being released upon exposure to water.
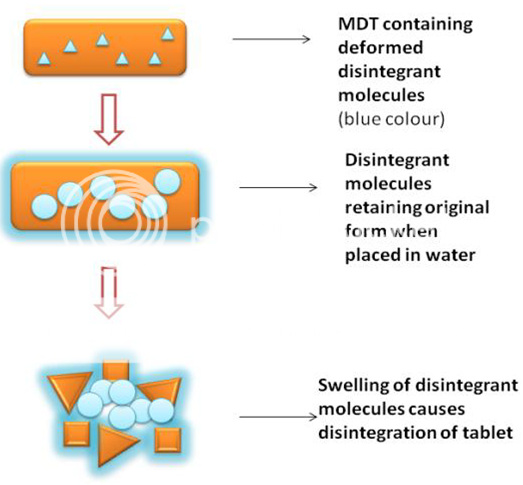
Figure 4: Deformation mechanism of Disintegrant. In this mechanism, irregular arrangement of molecules (in contact with medium) causes fast disintegration
Various manufacturing techniques for MDDDS include:
1. Lyophilization
2. Moulding
3. Direct Compression
4. Cotton Candy Process
5. Spray Drying
6. Sublimation
7. Mass Extrusion
8. Nanonization
9. Fast Dissolving Films
1.Freeze-Drying or Lyophilization[4 ,9,10]
In freeze-drying process, the water is sublimed from the product after it is frozen. This technique forms the basis of Zydis, Quicksolv and Lyoc technologies which are used to manufacture MDTs. Jaccard and Leyder used lyophilization to develop an oral formulation that not only dissolved rapidly but also exhibited improved bioavailability of several drugs such as spironolactone and trolendomycin. Corveleyn and Remon studied various formulation and process parameters by using hydrochlorothiazide as a model drug.
Zydis technology (ZT) is a patented technique, which had been used for drugs like famotidine, loperamide, piroxicam, oxazepam, lorazepam, domeperidone, brompheniramine, olanzepine, ondansetron and rizatriptan. Thirteen products are currently available in the market, which had been manufactured using this technology. In U.S., the MDT products available are: Claritin Reditab, Dimetapp Quick Dissolve, Feldene Melt, Maxalt- MLT, Pepcid RPD, Zofran ODT and Zyprexa Zydis. In the worldwide market, Zydis formulations are also available for oxazepam, lorazepam, loperamide, and enalapril. ZT utilizes a unique freeze-drying process to manufacture finished dosage units which significantly differ from conventional oral systems.
The process involves the following steps(Figure:5):
Stage 1 - bulk preparation of an aqueous drug solution or suspension and its subsequent precise dosing into pre-formed blisters. It is the blister that actually forms the tablet shape and is, therefore, an integral component of the total product package.
Stage 2 - passing the filled blisters through a specially designed cryogenic freezing process to control the ultimate size of the ice crystals which ensures that the tablets possess a porous matrix to facilitate the rapid disintegration property. These frozen units are then transferred to large-scale freeze dryers for the sublimation process, where the majority of the remaining moisture is removed from the tablets.
Stage 3 - Sealing the open blisters using a heat-seal process to ensure stability and protection of the product from varying environmental conditions.
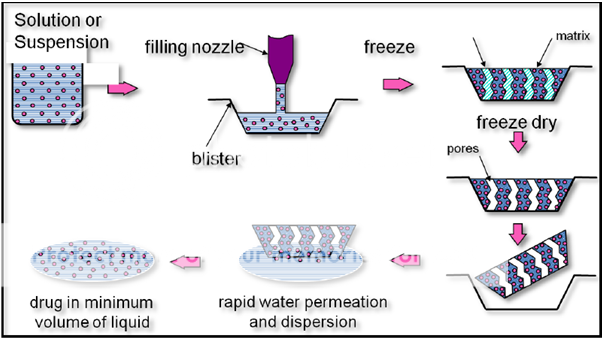
Figure 5: Lyophilization Technology. Patented technology based on this process is Zydis technology
Lyoc Lyoc technology lyophilizes, or “freeze-dries” an aqueous solution, suspension, or emulsion of an API and excipients. Lyoc’s high degree of porosity yields shorter disintegration times than compressed tablets. The Lyoc manufacturing process produces a stable product without use of additives, preservatives or gelatins. This process is environmentally friendly and cost-effective because it doesn’t require organic solvents. Lyoc technology is compatible with CIMA taste-masking techniques, customized release, high dosing and fixed-dose combination products.
Quicksolv[9,10] is a porous solid form obtained by freezing an aqueous dispersion/solution of the drug containing matrix and then drying it by removing the water using excess of alcohol (solvent extraction). The final form disintegrates very rapidly but is limited to low drug content and can be used only for those drugs that are insoluble in the extraction solvent. The ideal drug characteristics required for this technology are relative low aqueous solubility, fine particle size < 50 μm and good aqueous stability in the suspension.
The maximum drug loading capacity for water insoluble and soluble drugs are 400 mg and 60 mg, respectively. The primary problems associated with water soluble drugs are the formation of eutectic mixtures resulting in freezing-point depression and the formation of a glassy solid on freezing which might collapse on drying due to loss of supporting structure during sublimation process.
MDTs manufactured using lyophilization process, usually contain excipients like polymers (e.g., gelatin, alginates and dextrin) to provide strength and rigidity to tablets; polysaccharides (e.g., mannitol and sorbitol) to impart crystallinity and hardness to the matrix and to improve palatability; collapse protectants (e.g., glycine) to prevent the product from shrinking in its packaging during manufacturing or storage; flocculating agents (e.g., xanthan gum and acacia) to provide uniform dispersion of drug particles; preservatives (e.g., parabens) to prevent microbial growth; permeation enhancers (e.g.,sodium lauryl sulfate) to improve transmucosal permeability; pH adjusters (e.g. citric acid etc.) to optimize chemical stability; flavors and sweeteners to improve patient compliance and water to ensure formation of porous units.
Advantages
The major advantage of using this technique is that the tablets produced by this technology have very low disintegration time and have great mouthfeel due to fast melting effect.
Disadvantages
Although being a fairly routine process, lyophilization has some disadvantages like it is a relatively expensive and time consuming process. Furthermore, the product obtained is poorly stable and fragile, rendering conventional packaging unsuitable.
Tablet Moulding[9,11]
Moulded tablets invariably contain water-soluble ingredients due to which the tablets dissolve completely and rapidly. Following are the different tablet moulding techniques:
Compression Moulding Process
This manufacturing process involves moistening the powder blend with a hydroalcoholic solvent followed by pressing into mould plates to form a wetted mass (compression moulding). The solvent is then removed by air drying, a process similar to the manufacture of tablet triturates. Such tablets are less compact than compressed tablets and possess a porous structure that hastens dissolution.
Heat-Moulding Process
Heat-moulding process involves setting the molten mass containing a dispersed drug. This process uses agar solution as a binder and a blister packaging well as a mould to manufacture the tablet. A suspension containing drug, agar and sugar is prepared followed by pouring the suspension into the blister packaging well, solidifying the agar solution at room temperature to form a jelly and finally drying at approximately 30 °C under vacuum.
Moulding by Vacuum Evaporation without Lyophilization
This process involves pouring of the drug excipient mixture (in the form of a slurry or paste) into a mould of desired dimension, freezing the mixture to form a solidified matrix and finally subjecting it to vacuum drying at a temperature within the range of its collapse temperature and equilibrium freezing temperature. This results in the formation of a partially collapsed matrix. This method differs from the lyophilization technique, as in the former the evaporation of free unbound solvent occurs from a solid through the liquid phase to a gas, under controlled conditions, instead of the sublimation which takes place in the latter process. Unlike lyophilization, vacuum drying helps to densify the matrix and thereby improves the mechanical strength of the product. Pebley et al., evaporated the frozen mixture containing a gum (e.g., acacia, carageenan, guar, tragacanth or xanthan), a carbohydrate (e.g., dextrose, lactose, maltose, mannitol or maltodextrin) and solvent in a tablet-shaped mould to design a MDT with a disintegration time of about 20–60 secs.
Tablets produced by moulding are solid dispersions. The drug, depending on its solubility in the carrier, exists either as discrete particles or microparticles dispersed in the matrix and is dissolved totally/partially to form a solid solution/dispersion in the carrier matrix.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Advantages
As the dispersion matrix is made from water-soluble sugars, moulded tablets disintegrate more rapidly and offer improved taste. These properties are enhanced when tablets with porous structures are produced or when components that are physically modified by the moulding process are used. In comparison to lyophilization process, tablets produced by moulding technique are easier to adapt to the industrial scale.
Disadvantage
As the moulded tablets have poor mechanical strength, they may undergo erosion and breaking during handling. Though hardening can increase the strength of the tablets but it would be at the cost of their disintegration time.
Direct Compression (DC)[9,10,11]
DC is the simplest and most cost effective tablet manufacturing technique for MDTs as they can be fabricated using conventional tablet manufacturing and packaging machinery and also due to availability of tabletting excipients with improved flow, compressibility and disintegration properties, especially tablet disintegrants, effervescent agents and sugarbased excipients.
Table No.2 Ideal Requirements, Advantages and Limitations of Direct Compression
|
S. No |
Ideal requirements |
Advantages |
Limitations |
|
1 |
Flowability |
Cost effective production |
Segregation |
|
2. |
Compressibility |
Better stability of API |
Variation in functionality |
|
3. |
Dilution Potential |
Faster dissolution |
Low dilution potential |
|
4. |
Reworkability |
Less wear and tear of punches |
Reworkability |
|
5. |
Stability |
Simple validation |
Poor compressibility of API |
|
6. |
Controlled Particle Size |
Low microbial contamination |
Lubricant sensitivity |
Disintegrants
In many MDT products based on DC process, the disintegrants mainly affect the rate of disintegration and hence dissolution which is further enhanced in the presence of water soluble excipients and effervescent agents. The introduction of superdisintegrants has increased the popularity of this technology. Tablet disintegration time can be optimized by focusing on the disintegrant concentration.
Below a critical disintegrant concentration, tablet disintegration time becomes inversely proportional to disintegrant concentration. However, above the critical concentration level of disintegrant, disintegration time remains approximately constant or the decrease is insignificant.
Another DC based technology; Flashtab contains coated crystals of drug and microgranules alongwith disintegrants. In this technology, two types of disintegrants are used: a disintegrating agent (e.g., modified cellulose), which has a high swelling force and a swelling agent (e.g., starch) which has a low swelling force. Bi et al. and Watanbe. used microcrystalline cellulose (MCC) and low substituted hydroxypropyl cellulose (HPC) to manufacture MDTs wherein the ratio of MCC to HPC varied from 8:2 to 9:1. Ito and Sugihara investigated the application of agar powder as a disintegrant due to its property of absorbing water and considerable swelling without forming a gel at physiological temperature.
Effervescent Agents
The evolution of CO2 as a disintegrating mechanism forms the basis of the patented Orasolv technology (OT) and is frequently used to develop over-the-counter formulations. The product contains microparticles and is slightly effervescent in nature. Saliva activates the effervescent agent which causes the tablet to disintegrate. The OT had been utilized in fabrication of six marketed products: four Triaminic Softchew formulations, Tempra FirsTabs and Remeron SolTab.
The present technology uses the concept of effervescence to achieve fast-disintegration. In this technology, the microparticles are prepared by dispersing the drug into a suitable polymer (ethyl cellulose, methyl cellulose, acrylate or methacrylic acid resins) along with other excipients (mannitol and magnesium oxide). The drug and mannitol are added to the polymeric dispersion under stirring, followed by addition of magnesium oxide.
Here, mannitol and magnesium oxide are known as release promoters as they aid in drug release from the polymeric coating. This mixture is then dried for one hour at 50 °C, delumped and dried for another hour at the same temperature. The material is then screened (8-mesh) and dried for one hour at 60 °C. The formed microparticles, effervescent agents and other excipients are blended and compressed into tablets at 1.0–2.0 kp hardness. The tablets obtained are fragile with in-vivo disintegration time of less than one minute. As the tablets are very soft, they are packed into aluminium blisters using a specially designed packaging system. To reduce their friability, a novel method, known as particulate effervescent couple, had been developed. In this method the organic acid crystals are coated using a stoichiometrically low quantity of base material as compared to acid. The particle size of the organic acid crystals is carefully chosen to be greater than the base material so that base gets uniformly coated onto the acid crystals. The coating process is initiated by the addition of a reaction initiator, which in this case, is purified water. The reaction is allowed to proceed only to an extent of completion of base coating on organic acid crystals. The required end-point for the reaction termination is determined by measuring CO2 evolution. The resulting effervescent couple can be used in tablet preparation by mixing with polymer-coated drug particles and other required excipients.
Though, the Orasolv tablet has the appearance of a traditional compressed tablet, they are lightly compressed and are weaker and more brittle than the conventional tablets. Therefore, a special handling and packaging system for Orasolv was developed. An advantage of low degree of compaction is that the particle coating used for taste masking is not compromised by fracture during compression.
Durasolv, a second-generation technology was developed to produce robust MDTs. Durasolv has much higher mechanical strength than its predecessor due to use of higher compaction pressure during compression. It is thus produced in a much faster and costeffective manner and can be packed in either traditional blister packs or vials.
The limitations of Durasolv is its low drug loading capacity and high compaction pressure which are not suitable for incorporation of taste masked coated pellets. Therefore, the Durasolv technology is best suited for relatively small doses of drug. This technology has been applied in the fabrication of two products: NuLev and Zomig ZMT. However, the major drawback of effervescent excipients is their hygroscopicity which require control of humidity during processing and protection of the final product resulting in increase in the cost of the product.
Sugar-Based Excipients
Another approach to manufacture MDTs by DC is the use of sugar-based excipients (e.g., dextrose, fructose, isomalt, lactitol, maltitol, maltose, mannitol, sorbitol, starch hydrolysate, polydextrose and xylitol) which display high aqueous solubility and sweetness and hence, imparts taste masking and a pleasing mouth feel.
Mizumoto et al., have classified sugar-based excipients into two types based on their mouldability and dissolution rate.
Type I saccharides (e.g., lactose and mannitol) exhibit low mouldability but high dissolution rate.
Type II saccharides (e.g., maltose and maltitol) exhibit high mouldability but low dissolution rate.
Mouldability is defined as the capacity of the compound to be compressed/ moulded and to dissolve. It does not refer to the formation of a true mould by melting or solvent wetting process. The mouldability of Type I saccharide can be improved by granulating it with a Type II saccharide solution.
The above technology forms the basis of WOWTAB which involves the use of fluidized bed granulation for the surface treatment of Type I saccharide with Type II saccharide. This technique has been used in the production of Benadryl Fast melt tablets. Here, two different types of saccharides are combined to obtain a tablet formulation with adequate hardness and fast dissolution rate. Due to its significant hardness, the WOWTAB formulation is more stable to the environmental conditions than the Zydis or Orasolv and is suitable for both conventional bottle and blister packaging. The taste masking technology utilized in the WOWTAB is proprietary and claims to offer superior mouthfeel due to the patented smooth-melt action.
In the process of granulation, low mouldable sugar was coated with high mouldable sugar followed by a specific humidity treatment, to achieve fast disintegration. The resulting tablet had a hardness of 1.0–2.0 kg (tablet-size dependent) and presented a preferable disintegration time of 1–40 secs. Various classes of drugs can be incorporated into the above sugar combination to achieve a MDT with optimum performance characteristics. A preferable ratio of 5–10% w/w of high mouldable sugar was found to be sufficient to achieve the desired hardness and disintegration property.
A series of experiments had been conducted to develop a MDT using a combination of starch/cellulose and one or more water-soluble saccharides. Erythritol was found to be the best saccharide because it displayed rapid disintegration, good tolerability, sweetening and a refreshing mouth feel due to its negative heat of solution.
Recently, the Ziplet technology was developed, which can be used for water insolubledrugs or drugs as coated microparticles. It was found that the addition of a suitable amount of a water-insoluble inorganic excipient combined with one or more effective disintegrants imparted an excellent physical resistance to the MDT and simultaneously maintained optimal disintegration even at low compression force and tablet hardness . In fact, breakage of the tablet edges or formation of powder during manufacturing and opening of the blister pack is avoided because of its superior mechanical resistance. The use of water-insoluble inorganic excipients also offers better enhancement of disintegration characteristics in comparison to the most commonly used water-soluble sugars or salts. In fact, tablets composed primarily of water-soluble components often tend to dissolve rather than disintegrate, resulting in much longer disintegration time. As the soluble components dissolve on the tablet’s outer layer, a concentrated viscous solution is formed, which reduces the rate of water diffusion into the tablet core.
Cotton Candy Process[3,11,12]
The FLASHDOSE® is a MDDDS manufactured using Shearform™ technology in association with Ceform TI™ technology to eliminate the bitter taste of the medicament . The Shearform technology is employed in the preparation of a matrix known as ‘floss’, made from a combination of excipients, either alone or with drugs. The floss is a fibrous material similar to cotton-candy fibers, commonly made of saccharides such assucrose, dextrose, lactose and fructose at temperatures ranging between 180–266 °F. However, other polysaccharides such as polymaltodextrins and polydextrose can be transformed into fibers at 30–40% lower temperature than sucrose. This modification permits the safe incorporation of thermolabile drugs into the formulation. The tablets manufactured by this process are highly porous in nature and offer very pleasant mouthfeel due to fast solubilization of sugars in presence of saliva. The manufacturing process can be divided into four steps as detailed below.
I. Floss Blend
In this step, 80% sucrose in combination with mannitol/dextrose and 1% surfactant is blended to form the floss mix. The surfactant acts as a crystallization enhancer in maintaining the structural integrity of the floss fibers. It also helps in the conversion of amorphous sugar into crystalline form from an outer portion of amorphous sugar mass and subsequently converting the remaining portion of the mass to complete crystalline structure. This process helps to retain the dispersed drug in the matrix, thereby minimizing migration out of the mixture.
II. Floss Processing
The floss formation machine uses flash heat and flash flow processes to produce matrix from the carrier material. The machine is similar to that used in ‘cotton-candy’ formation which consists of a spinning head and heating elements. In the flash heat process, the heat induces an internal flow condition of the carrier material. This is followed by its exit through the spinning head (2000–3600 rpm) that flings the floss under centrifugal force and draws into long and thin floss fibers, which are usually amorphous in nature.
III. Floss Chopping and Conditioning
This step involves the conversion of fibers into smaller particles in a high shear mixergranulator. The conditioning is performed by partial crystallization through an ethanol treatment (1%) which is sprayed onto the floss and subsequently evaporated to impart improved flow and cohesive properties to the floss.
IV. Blending and Compression
Finally, the chopped and conditioned floss fibers are blended with the drug alongwith other required excipients and compressed into tablets. In order to improve the mechanical strength of the tablets, a curing step is also carried out which involves the exposure of the dosage forms to elevated temperature and humidity conditions, (40 °C and 85% RH for 15 min). This is expected to cause crystallization of the floss material that results in binding and bridging to improve the structural strength of the dosage form .
Spray-Drying[3,12]
Allen et al., have used spray-drying for the production of MDTs. The formulations contained hydrolyzed and unhydrolyzed gelatin as a supporting agent for the matrix, mannitol as a bulking agent and sodium starch glycolate/croscaramellose as a disintegrant. Disintegration and dissolution were further enhanced by adding an acid (e.g., citric acid) or an alkali (e.g., sodium bicarbonate). The suspension of above excipients was spray-dried to yield a porous powder which was compressed into tablets. Tablets manufactured by this method disintegrated in < 20 secs in an aqueous medium.
Sublimation[13,19]
Sublimation has been used to produce MDTs with high porosity. A porous matrix is formed by compressing the volatile ingredients alongwith other excipients into tablets, which are finally subjected to a process of sublimation(figure 6). Inert solid ingredients with high volatility (e.g., ammonium bicarbonate, ammonium carbonate, benzoic acid, camphor, hexamethylene tetramine, naphthalene, phthalic anhydride, urea and urethene) have been used for this purpose. Solvents such as cyclohexane and benzene were also suggested for generating the porosity in the matrix. Makino et al., reported a method using water as a pore-forming material.
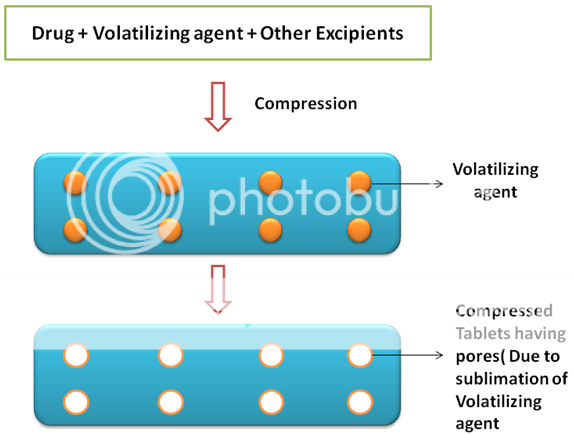
Figure 6: Sublimation technique. Evaporation of volatile agent results in formation of porous tablets there by causing fast disintegration
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Mass-Extrusion[17]
This technology involves softening of the active blend using the solvent mixture of water soluble polyethylene glycol and methanol and expulsion of softened mass through the extruder or syringe to get a cylindrical shaped extrude which are finally cut into even segments using heated blade to form tablets. This process can also be used to coat granules of bitter drugs to mask their taste.
Nanonization[17]
A recently developed Nanomelt technology involves reduction in the particle size of drug to nanosize by milling the drug using a proprietary wet-milling technique . The nanocrystals of the drug are stabilized against agglomeration by surface adsorption on selected stabilizers, which are then incorporated into MDTs. This technique is especially advantageous for poorly water soluble drugs. Other advantages of this technology include fast disintegration/dissolution of nanoparticles leading to increased absorption and hence higher bioavailability and reduction in dose, cost effective manufacturing process, conventional packaging due to exceptional durability and wide range of doses (up to 200 mg of drug per unit).
Fast Dissolving Films[19]
It is a new frontier in MDDDS that provides a very convenient means of taking medications and supplements. In this technique, a non-aqueous solution is prepared containing water soluble film forming polymer (pullulan, carboxy methylcellulose, hydroxypropyl methylcellulose, hydroxyl ethylcellulose, hydroxyl propylcellulose, polyvinyl pyrrolidone, polyvinyl alcohol or sodium alginate, etc.), drug and other taste masking ingredients, which is allowed to form a film after evaporation of solvent. In case of a bitter drug, resin adsorbate or coated microparticles of the drug can be incorporated into the film. This film, when placed in mouth, melts or dissolves rapidly, releasing the drug in solution or suspension form. The features of this system include paper thin films of size less than 2X2 inches, dissolution in 5 sec, instant drug delivery and flavoured after taste.
Patented Technologies for preparation of MDT:[3,4,11,13,18]
Several technologies are available for preparing Mouth dissolving tablets. But some commercially useful technologies are:
Zydis technology: ‘Zydis’ is the first mouth dissolving dosage form in the market. It is a unique freeze?dried tablet in which the active drug is incorporated in a water?soluble matrix, which is then transformed into blister pockets and freeze dried to remove water by sublimation. Zydis matrix is made up of a number of ingredients in order to obtain different objectives. Polymers such as gelatin, dextran or alginates are added to impart strength during handling. These form a glossy and amorphous structure. Mannitol or sorbitol is added to impart crystallinity, elegance and hardness. Various gums may be added to prevent sedimentation of dispersed drug particles. Water is used as a medium to ensure the formation of a porous dosage form. Collapse protectants like glycine may be used to prevent shrinkage of dosage form during freeze drying and longterm storage.36 If necessary, suspending agents and pH adjusting agents may be used. Preservatives may also be added to prevent microbial growth. Zydis products are packed in blister packs to protect the formulation from environmental moisture. A secondary moisture proof foil punch is often required as this dosage form is very moisture sensitive. When put into the mouth, Zydis unit quickly disintegrates and dissolves in saliva.
Drawbacks:
a. A water insoluble drug can be incorporated only upto 400 mg per tablet or less. On the other hand water soluble drug can be incorporated only upto 60 mg
b. Fragility and poor stability of dosage form during storage under stressful conditions.
Orasolv technology: It is CIMA lab’s first mouth dissolving formulation. This technology involves taste masking of active drug. Effervescent disintegrating agent is also used. Conventional blenders and tablet equipments are used for preparation of tablets. Less force of compaction is used for manufacturing so as to obtain soft and quickly disintegrating tablets. There is a limitation of this technology that soft and fragile tablets are formed, therefore needed to be packed in specially designed pick and place package system.
Durasolv Technology: Durasolv is the patented technology of CIMA labs. The tablets made by this technology consist of drug, filler and a lubricant. Tablets are prepared by using conventional tabletting equipment and have good rigidity. These can be packaged into conventional packaging system like blisters. Durasolv is an appropriate technology for product requiring low amounts of active ingredients.
Wowtab technology: Yamanauchi pharmaceutical company patented this technology. ‘wow’ means ‘without water’. The active ingredients may constitute upto 50% w/w of the tablet. In this technique, saccharides of both low and high mouldability are used to prepare the granules. Mouldability is the capacity of a compound to be compressed. Highly mouldable substance has high compressibility and thus shows slow dissolution. The combination of high and low mouldability is used to produce tablets of adequate hardness. Active ingredients are mixed with low mouldability saccharides and then granulated with high mouldabiity saccharides and then compressed into tablet. The Wowtab product dissolves quickly in 15 s or less. Wowtab product can be packed in both into conventional bottle and blister packs.
Lyoc Technology: Oil in water emulsion is prepared and placed directly into blister cavities followed by freeze-drying. Non-homogeneity during freeze drying is avoided by incorporating inert filler to increase the viscosity.
Quick solv Technology: This technology uses two solvents in formulating a matrix, which disintegrates instantly. Methodology includes dissolving matrix components in water and the solution or dispersion is frozen. Then dry the matrix by removing water using excess of alcohol (solvent extraction). Thus the product formed has uniform porosity and adequate strength for handling.
FlashTab Technology: Flash dose technology has been patented by fuisz. Nurofen meltlet, a new form of ibuprofen as melt in mouth tablets prepared using flash dose technology is the first commercial product launched by biovail corporation. Flash dose tablets consist of self-binding shear form matrix termed as “floss”. Shear form matrices are prepared by flash heat processing. This technology involves the preparation of rapidly disintegrating tablet, which consists of an active ingredient in the form of microcrystal. Drug micro granules may be prepared by using the conventional techniques like coacervation, micro encapsulation, extrusionspheronization or simple pan coating method. The micro crystals or micro granules of the active ingredients are added to the granulated mixture of excipients prepared by wet or dry granulation and compressed into tablets.
Shearform Technology: In this technology, a shearform matrix, ‘Floss’ is prepared. Feedstock prepared with a sugar carrier is subjected to flash heat processing. In this process, sugar is simultaneously subjected to centrifugal force and to a temperature gradient, which causes the temperature of the mass to rise and hence an internal flow condition is created, permitting part of it to move with respect of the mass. The flowing mass comes out through the spinning head that flings the floss. The produced floss is amorphous in nature. So by various techniques, it is further chopped and recrystallised to provide a uniform flow, thus facilitate blending. Then the recrystallised matrix, active drug and other excipients are blended together and finally compressed into tablets. Active drug and other excipients may be blended with the floss before recrystallising it.
Dispersible tablet Technology: It offers development of ODT with improved dissolution rate by incorporating 8-10% of organic acids and disintegrating agents. Disintegrate include starch, modified starches, microcrystalline cellulose, alginic acid, cross-linked sodium carboxymethyl cellulose and cyclodextrins.
Pharma burst Technology: It utilizes the coprocessed excipients to develop ODT, which dissolves within 30-40s.This technology involves dry blending of dug, flavor, lubricant followed by compression into tablets.
Frosta Technology: Akina patents this technology. It utilizes the concept of formulating plastic granules and co processing at low pressure to produce strong tablets with high porosity. Plastic granules composed of:
1. Porous and plastic material
2. Water penetration enhancer, and Binder
The process involves usually mixing the porous plastic material with water penetration enhancer and followed by granulating with binder. The tablets obtained have excellent hardness and rapid disintegration time ranging from 15 to 30s depending on size of tablet .
Oraquick Technology: It utilizes taste masking microspheres technology called as micro mask, which provides superior mouth feel, significant mechanical strength, and quick disintegration/dissolution of product. This form of matrix that protects drug, which can be compressed with sufficient mechanical strength. Oraquick product dissolves within few seconds.
Ziplets/Advatab Technology: It utilizes waterinsoluble ingredient combined with one or more effective disintegrants to produce ODT with improved mechanical strength and optimal disintegration time at low compression force.
Cotton candy technology: It is patented by Fuisz. Cotton candy technology utilizes a unique spinning mechanism to produce floss like crystalline structure. This crystalline sugar can incorporate the active drug into a tablet. A final product has a very high surface area for dissolution. Once placed on the tongue it disperses and dissolves quickly.
Nanocrystal technology
This is patented by Elan, King of Prussia. Nanocrystal technology includes lyophilization of colloidal dispersions of drug substance and water soluble ingredients filled into blister pockets. This method avoids manufacturing process such as granulation, blending and tabletting which is more advantages for highly potent and hazardous drugs. As manufacturing losses are negligible, this process is useful for small quantities of drug .
Table-3 List of commercially Available Fast dissolving tablets
|
Trade name |
Active drug |
Manufacturer |
|
Felden fast melt |
Piroxicam |
Pfiser Inc., NY, USA |
|
Claritin redi Tab |
Loratidine |
Schering plough Corp., USA |
|
Maxalt ML |
Rizatriptan |
Merck and Co., NJ, USA |
|
Zyprexia |
Olanzapine |
Eli lilly, Indianapolis, USA |
|
Pepcid RPD |
Famotidine |
Merck and Co., NJ, USA |
|
Zofran ODT |
Ondansetron |
Glaxo Wellcome, Middlesex, UK |
|
Zoming-ZMT |
Zolmitriptan |
AstraZeneca, Wilmington, USA |
|
Zeplar TM |
Selegilline |
Amarin Corp., London, UK |
|
Tempra Quiclets |
Acetaminophen |
Bristol myers Squibb, NY, USA |
|
Febrectol |
Paracetamol |
Prographarm, Chateauneuf, France |
|
Nimulid MDT |
Nimesulide |
Panacea Biotech, New delhi , India |
|
Torrox MT |
Rofecoxib |
Torrent pharmaceuticals , India |
|
Olanex instab |
Olanzapine |
Ranbaxy lab. Ltd. New-delhi, India |
|
Benadryl Fastmelt |
Diphenhydramine and pseudoephedrine |
Warner Lambert, NY, USA |
EVALUATION PARAMETERS:[13,14,15,19]
Weight variation test: Randomly selected 20 tablets were taken and their individual weights & the average weight of 20 tablets were determined. The deviation of each individual tablet from the average weight was calculated and compared with the standard values given in Pharmacopoeia.
The % weight variation of each individual tablet from the average weight is calculated by the given formula
![]()
Hardness test: Hardness of the tablets was measured by using hardness testers like Monsanto hardness tester, Pfizer hardness tester etc. The pressure required to break the tablets is measured as a function of hardness ( kg/ cm2 ). The values obtained must meet the standard value.
Friability: Friability is to measure the extent of tablet breakage during physical stress conditions like Packing, transportation etc. A sample of randomly selected 6 tablets was evaluated for friability using Roche friabilator at 25 rpm for 4 minutes. The % weight loss is calculated by measuring the total weight of 6 tablets before and after operation. Formula for calculating the % weight loss is given below:
![]()
Wetting time:
Wetting time and water absorption ratio are the significant parameters for mouth dissolving tablets. The following method for calculating the wetting time of the tablet. A piece of filter paper (circularly cut) was placed in a small petri plate containing water soluble dye solution. Tablet was placed on the paper and the time required for complete wetting of the tablet was determined (Figure 7).Bi Y. et al. used a tissue paper folded twice and was placed in a small culture dish ( i.d = 6.5 cm) containing 6 ml of water.
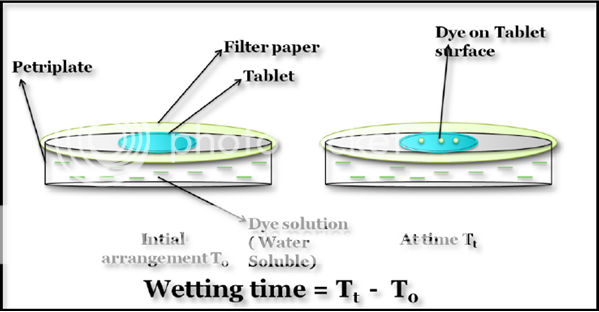
Figure 7: Wetting time of Mouth dissolving tablet. The time taken for appearance of dye colour on tablet is wetting time
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Water absorption ratio:
Similar to the procedure followed in determination of wetting time (Figures 8). However, here the initial weight and the final weight (after complete wetting) of tablet were calculated and the water absorption ratio was calculated by given formula:
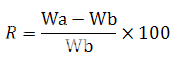
Where, R is water absorption ratio, Wa and Wb are the weights of tablet before and after wetting respectively.
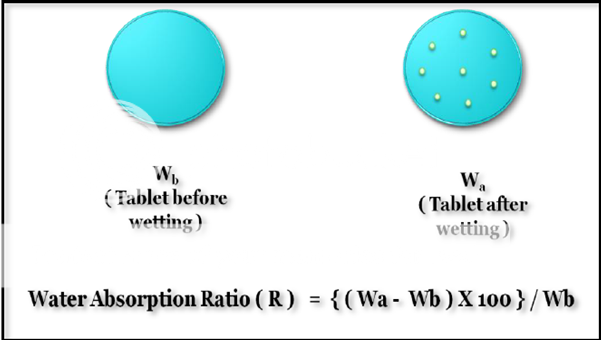
Figure 8: Calculation of water absorption ratio for MDTs. Difference between intial and final weights of tablet is noted Water absorption
Disintegration time: Disintegration time for randomly selected 6 tablets was measured using disintegration test apparatus. The average time required for disintegration was calculated and compared with standards.
Invitro dissolution studies: Randomly selected 6 tablets were subjected to drug release studies using USP dissolution apparatus, in dissolution medium volume of 900 ml was used and a temperature of 37±0.5oC was maintained. 5 ml of the sample was collected for every 5 minutes interval till 30 minutes and replaced with 5 ml of fresh buffer solution. The samples were filtered and suitably diluted and the drug assay was performed using UV spectrophotometer or HPLC system. The results were compared with standard values.
Taste or mouth feel: Healthy human volunteers were used for evaluation of mouth feel of the tablet. One tablet was evaluated for its mouth feel. A panel of 5 members evaluate the mouth feel by time intensity method. Sample equivalent to 40 mg was held in mouth for 10 seconds and the opinion is rated by giving different score values. (0: good, 1: tasteless, 2: slightly bitter, 3: bitter, 4: awful).
Stability studies: Various stability studies like accelerated stability study, intermediate and long term stability studies were done during preformulation. The sample was subjected to higher temperature or humidity or both, to know their impact on the stability of mouth dissolving tablet.
Uniformity of dispersion: Two randomly selected tablets were kept in 100 ml water and stirred for two minutes. The dispersion was passed through 22 meshes. The tablets were considered to pass the test if no residue remains on the screen.
Drugs to be promising in corporate in Mouth dissolving tablets[16,17,18,20,21]
There are no particular limitations as long as it is a substance which is used as a pharmaceutical active ingredient.
Analgesics and Anti-inflammatory Agents:
Aloxiprin, Auranofin, Azapropazone, Benorylate, Diflunisal, Etodolac, Fenbufen, Fenoprofen Calcim, Flurbiprofen, Ibuprofen, Indomethacin, Ketoprofen, Meclofenamic Acid, Mefenamic Acid, Nabumetone, Naproxen, Oxaprozin, Oxyphenbutazone, Phenylbutazone, Piroxicam, Sulindac.
Anthelmintics :
Albendazole, Bephenium Hydroxynaphthoate, Cambendazole, Dichlorophen, Iverrnectin, Mebendazole, Oxarnniquine, Oxfendazole, Oxantel Embonate, Praziquantel, Pyrantel Embonate, Thiabendazole.
Anti-Arrhythmic Agents:
Amiodarone, Disopyramide, Flecainide Acetate, Quinidine Sulphate,
Anti-bacterial Agents:
Benethamine Penicillin, Cinoxacin, Ciprofloxacin, Clarithromycin, Clofazimine, Cloxacillin, Demeclocycline, Doxycycline, Erythromycin, Ethionamide, Imipenem, Nalidixic Acid, Nitrofurantoin, Rifampicin, Spiramycin, Sulphabenzamide, Sulphadoxine, Sulphamerazine, Sulphacetamide, Sulphadiazine, Sulphafurazole, Sulphamethoxazole, Sulphapyridine, Tetracycline, Trimethoprim.
Anti-coagulants:
Dicoumarol, Dipyridamole, Nicoumalone, Phenindione. Anti-Depressants: Amoxapine, Ciclazindol, Maprotiline, Mianserin, Nortriptyline, Trazodone, Trimipramine Maleate., Acetohexamide, Chlorpropamide, Glibenclamide, Gliclazide, Glipizide, Tolazamide, Tolbutamide.
Anti-Epileptics:
Beclamide, Carbamazepine, Clonazepam, Ethotoin, Methoin, Methsuximide, Methylphenobarbitone, Oxcarbazepine, Paramethadione, Phenacemide, Phenobarbitone, Phenytoin, Phensuximide, Primidone, Sulthiame, Valproic Acid.
Anti-Fungal Agents:
Amphotericin, Butoconazole Nitrate, Clotrimazole, Econazole Nitrate, Fluconazole, Fiucytosine, Griseofulvin, Itraconazole, Ketoconazole, Miconazole, Natamycin, Nystatin, Sulconazole Nitrate, Terbinafine, Terconazole, Tioconazole, Undecenoic Acid.
Anti-Gout Agents:
Allopurinol, Probenecid, Sulphinpyrazone.
Anti-Hypertensive Agents:
Amlodipine, Carvedilol, Benidipine, Darodipine, Dilitazem, Diazoxide, Felodipine, Guanabenz Acetate, Indoramin, Isradipine, Minoxidii, Nicardipine, Nifedipine, Nimodipine, Phenoxybenzamine, Prazosin, Reserpine, Terazosin.
Anti-Malarials:
Amodiaquine, Chloroquine, Chlorproguanil, Halofantrine, Mefloquine, Proguanil, Pyrimethamine, Quinine Sulphate. Anti-Migraine Agents: Dihydroergotamine Mesyiate, Ergotamine Tartrate, Methysergide Maleate, Pizotifen Maleate, Sumatriptan Succinate.
Anti-Muscarinic Agents:
Atropine, Benzhexol, Biperiden, Ethopropazine, Hyoscine Butyl Bromide, Hyoscyarnine, Mepenzolate Bromide, Orphenadrine, Oxyphencylcimine, Tropicamide.
Anti-Neoplastic Agents and Immunosuppressants:
Aminoglutethimide, Amsacrine, Azathiopnne, Busulphan, Chlorambucil, Cyclosporin, Dacarbazine, Estramustine, Etoposide, Lomustine, Melphalan, Mercaptopurine, Methotrexate, Mitomycin, Mitotane, Mitozantrone, Procarbazine, Tamoxifen Citrate, Testolactone.
Anti Protozoal Agents:
Benznidazole, Clioquinol, Decoquinate, Diiodohydroxyquinoline, Diloxanide Furoate, Dinitolmide, Furzolidone, Metronidazole, Nimorazole, Nitrofurazone, Omidazole, Tinidazole.
Anti-Thyroid Agents:
Carbimazole, Propylthiouracil.
Anxiolytic, Sedatives, Hypnotics and Neuroleptics:
Alprazolam, Amyiobarbitone, Barbitone, Bentazeparn, Bromazepam, Bromperidol, Brotizoiam, Butobarbitone, Carbromal, Chlordiazepoxide, Chlormethiazole, Chlorpromazine, Clobazam, Clotiazepam, Clozapine, Diazepam, Droperidol, Ethinamate, Flunanisone, Flunitrazepam, Fluopromazine, Flupenuiixol Decanoate, Fluphenazine Decanoate, Flurazepam, Haloperidol, Lorazepam, Lormetazepam, Medazepam, Meprobamate, Methaqualone, Midazolam, Nitrazepam, Oxazepam, Pentobarbitone, Perphenazine Pimozide, Prochlorperazine, Suipiride, Temazepam, Thioridazine, Triazolam, Zopiclone.
Cardiac Inotropic Agents:
Amrinone, Digitoxin, Digoxin, Enoximone, Lanatoside C, Medigoxin.
Corticosteroids:
Beclomethasone, Betamethasone, Budesonide, Cortisone Acetate, Desoxymethasone, Dexamethasone, Fludrocortisone Acetate, Flunisolide, Flucortolone, Fluticasone Propionatu, Hydrocortisone, Methylprednisolone, Prednisolone, Prednisone, Triamcinolone.
Diuretics:
Acetazolarnide, Amiloride, Bendrofluazide, Bumetanide, Chlorothiazide, Chlorthalidone, Ethacrynic Acid, Frusemide, Metolazone, Spironolactone, Triamterene.
Anti-Parkinsonian Agents:
Bromocriptine Mesylate, Lysuride Maleate.
Gastro-Intestinal Agents:
Bisacodyi, Cimetidine, Cisapride, Diphenoxylate, , Domperidone, Famotidine, Loperamide, Mesalazine, Nizatidine, Omeprazole, Ondansetron, Ranitidine, Sulphasaiazine.
Histamine H,-Receptor Antagonists:
Cetrizine, Astemizole, Cinnarizine, Cyclizine, Cyproheptadine, Dimenhydrinate, Flunarizine, Loratadine, Meclozine, Oxatomide, Terfenadine, Triprolidine.
Lipid Regulating Agents:
Bezafibrate, Clofibrate, Fenofibrate, Gemfibrozil, Probucol.
Local Anaesthetics:
Lidocaine
Neuro -Muscular Agents:
Pyridostigmine.
Nitrates and Other Anti-Anginal Agents:
Amyl Nitrate, Glyceryl Trinitrate, Isosorbide Dinitrate, Isosorbide Mononitrate, Pentaerythritol Tetranitrate.
Nutritional Agents:
Betacarotene, Vitamin A, Vitamin B 2 , Vitamin D, Vitamin E, Vitamin K.
Opioid Analgesics:
Codeine, Dextropropyoxyphene, Diamorphine, Dihydrocodeine, Meptazinol, Methadone, Morphine, Nalbuphine, Pentazocine.
Oral Vaccines:
Vaccines designed to prevent or reduce the symptoms of diseases of which the following is a Representative Influenza, Tuberculosis, Meningitis, Hepatitis, Whooping Cough, Polio, Tetanus, Diphtheria, Malaria, Cholera, Herpes, Typhoid, HIV, Aids, Measles, Lyme Disease, Travellers Diarrhea, Hepatitis A, B And C, Otitis Media, Dengue Fever, Rabies, Parainfluenza, Rubella, Yellow Fever, Dysentery, Legionnaires Disease, Toxoplasmosis, Q-Fever, Haemorrhegic Fever, Argentina Haemorrhagic Fever, Caries, Chagas Disease, Urinary Tract Infection Caused By E.Coli, Pneumoccoccal Disease, Mumps, File://H:\Gits Mdt\Fast Dissolving Tablet The Future Of Compaction And Chikungunya.
Proteins, Peptides and Recombinant Drugs:
Insulin (Hexameric/Dimeric/Monomeric Forms), Glucagon, Growth Hormone (Somatotropin), Polypeptides or Their Derivatives, (Preferably With A Molecular Weight from 1000 To 300,000), Calcitonins And Synthetic Modifications Thereof, Enkephalins, Interferons (Especially Alpha-2 Inter Feron For Treatment Of Common Colds).
Sex Hormones:
Clomiphene Citrate, Danazol, Ethinyloestradiol, Medroxyprogesterone Acetate, Mestranol, Methyltestosterone, Norethisterone, Norgestrel, Oestradiol, Conjugated Oestrogens, Progesterone, Stanozolol, Stiboestrol, Testosterone, Tibolone.
Conclusion:
The FDTs have potential advantages over conventional dosage forms, with their improved patient compliance, convenience, bioavailability and rapid onset of action had drawn the attention of many manufactures over a decade. FDTs formulations obtained by some of these technologies have sufficient mechanical strength, quick disintegration/dissolution in the mouth without water.There is a clear opportunity for new enhanced oral products arising within this market segment. Approximately one-third of the population, primarily the geriatric and pediatric populations, has swallowing difficulties, resulting in poor compliance with oral tablet drug therapy which leads to reduced overall therapy effectiveness. A new tablet dosage format, the fast dissolving tablet has been developed which offers the combined advantages of ease of dosing and convenience of dosing in the absence of water or fluid. These tablets are designed to dissolve or disintegrate rapidly in the saliva generally within <60 seconds (range of 5- 50seconds). The development of a fast-dissolving tablet also provides an opportunity for a line extension in the marketplace, A wide range of drugs (e.g., neuroleptics, cardiovascular drugs, analgesics, antihistamines, and drugs for erectile dysfunction) can be considered candidates for this dosage form.Pharmaceutical marketing is another reason for the increase in available fast dissolving/ disintegrating products. As a drug entity nears the end of its patent life, it is common for pharmaceutical manufacturers to develop a given drug entity in a new and improved dosage form. A new dosage form allows a manufacturer to extend market exclusivity, while offering its patient population a more convenient dosage form or dosing regimen.
Reference:
1) Kaur et al., Mouth dissolving tablets: A novel approach to drug delivery., International Journal of Current Pharmaceutical Research, 20011, 3, 1-7.
2) Seong Hoon Jeong, Kinam Park., Material properties for making fast dissolving tablets by a compression method.,Journal of Materials Chemistry., 2008, 18, 3527–3535.
3) McLaughlin Rosie,Banbury Susan,Crowley Kieran., Orally Disintegrating Tablets The Effect of Recent FDA Guidance on ODT Technologies and Applications.,Pharmaceutical Technology:Sep.(2009)
4) D. Shukla et al., Mouth Dissolving Tablets I: An Overview of Formulation Technology., Scientia Pharmceutica. 2009; 76; 309–326.
5) Hirani et al., Orally Disintegrating Tablets: A Review.,Tropical Journal of Pharmaceutical Research, April 2009; 8 (2): 163
6) Rish RK et al., A review on fast dissolving tablets techniques. The Pharma Review 2004; 2: 32.
7) Kuchekar BS, Atul, Badhan C, Mahajan HS., Mouth dissolving tablets: A novel drug delivery system., PharmaTimes 2003; 35: 7-9.
8) Bhaskaran S, Narmada GV. Rapid dissolving tablets a novel dosage form. Indian Pharmacist 2002; 1: 9–12.
9) H . Seager., Drug-delivery Products and the Zydis Fast-dissolving Dosage Form., J. Pharm. Pharmacol. 1998. 50: 375-382.
10) Vummaneni. V et. al., Mouth Dissolving Tablets: A Review., American Journal of Pharmatech Research., 2012; 2(3).
11) D Bhowmik et al., Fast Dissolving Tablet: An Overview., Journal of Chemical and Pharmaceutical Research, 2009, 1(1): 163-177
12) Bhupendra G Prajapati et al ., A Review on Recent patents on Fast Dissolving Drug Delivery System., International Journal of PharmTech Research.2009,1(3)
13) Jagani et.al.,Fast Dissolving Tablets: Present and Future Prospectus., Journal of Advances in Pharmacy and Healthcare Research.,2011, 2(1):57-70.
14) Gupta Kumar Alok, Mittal Anuj and Prof.Jha.K.K.,Fast Dissolving Tablet- A Review., The Pharma Innovation., 2012,1(1):1-8.
15) V.Dinesh kumar et al., A comprehensive review on fast dissolving tabletTechnology., Journal of Applied Pharmaceutical Science 01 (05); 2011: 50-58
16) D Bhowmik et al., Fast dissolving tablet: A review on revolution of novel drug delivery system and new market opportunities., Scholars Research Library., 2009, 1 (2) 262-276
17) Sharma.S.et al., Pharmainfo.net, 2008; 6(5). Available at: pharmainfo.net/reviews.
18) Rakesh Pahwa et al., Orally Disintegrating Tablets - Friendly to Pediatrics and Geriatrics., Archives of Applied Science Research., 2 (2): 35-48.
19) Abdul Sayeed et al., Mouth dissolving tablets: An Overview., International Journal of Research in Pharmaceutical and Biomedical Sciences, 2011,2(3): 959-970.
20) Debjit Bhowmik et al., Fast dissolving tablet: A review on revolution of novel drug delivery system and new market opportunities., Der Pharmacia Lettre, 2009, 1 (2) 262-276.
21) Deepak et al.,Fast disintegrating tablets: A new era in novel drug delivery system and new market opportunities., Journal of Drug Delivery & Therapeutics; 2012, 2(3): 74-86.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









