{ DOWNLOAD AS PDF }
About Authors:
Languluri Reddenna
Pharm-D, Department of Pharmacy Practice,
Rajiv Gandhi Institute of Medical Sciences, Kadapa, Andhra Pradesh, India-516003
reddennapharmd@gmail.com
Abstract
Objective Monitoring the safety of medicine use in children is of superlative prominence, on the other hand only limited data on this aspect. In-between 1979 and 2006, the poisoning death rate cut in half, declining from 0.35 to 0.17 per 100,000 children. Therefore, the main aim of the study was to evaluate the medication safety in children’s.
Methods A prospective observational study was conducted in the PICU, NICU, IP and OP department of Paediatrics at Rajiv Gandhi Institute of Medical Sciences overa 6-months period(from august 2012 to January 2013). The Institutional Ethics and Research Committee of Rajiv Gandhi Institute of Medical Sciences, Kadapa (Rc.No.413/Acad./2011-12), approved the study. All patients under 12 year of age were included in the study. Patients referred from another health facility or hospital to collect their medications from the pharmacy of the respective health clinics were excluded from the study. All documented medication orders monitored for any drug misadventures and recommended to the pediatricians.
Results Drug misadventures include medication errors 588 (84%), ADEs 27 (3.8%), ADRs 33 (4.8%) and drug interactions 52 (7.4%).
Conclusion Prescriber’s workload, lack of sufficient knowledge and time spent with one patient are restrains that lead to most of the mistakes. Recognition of these errors is the first step in their prevention. There is a crucial need to develop methods to reduce medication errors in children should be the major priority. It can be concluded that drug misadventures are common in children.
REFERENCE ID: PHARMATUTOR-ART-2123
Introduction
The objective of drug therapy is the attainment of distinct therapeutic outcomes that progress a patient’s quality of life while diminishing patient threat. With each therapy there must be a threat, it could be notorious or mysterious[1]. Monitoring the safety of medicine use in children is of superlative prominence, on the other hand only limited data on this aspect[2]. These threats demarcated as drug misadventures, which include both adverse drug reactions (ADRs) & medication errors. Deficiency of confirmation on the use of medicines in children pointers to improbability in dosing and escalations the threat of medication errors. Medication errors arise in children at analogous rates to adults however; errors had three times the expected to cause injury[3]. Neonates is a susceptible crowd for dosing and dispensing errors because of rapidly changing body surface area and weight, a rapidly evolving system of drug absorption, metabolism and excretion, an incompetence to converse with the source and off-label or unconstrained drug usage. Most of the drugs used in neonates desires a fraction of calculation and there by budding for errors. Medication errors in children are reported in literature; but to our knowledge, there are a very few studies that focus on medication errors in neonatology but none from India[4].
Amongst young children, a child intake medication while unverified causes 95% of unpremeditated medication overdose stays to emergency departments and roughly, 5% are due to dosing errors through by caregivers[5]. Fifty-six children years 14 and under expire each year from unpremeditated medication overdoses. In-between 1979 and 2006, the poisoning death rate cut in half, declining from 0.35 to 0.17 per 100,000 children. Yet, among all child-poisoning deaths, the number attributable to medications increased from 36% to 64%. Medicine classes most commonly associated with error in primary care include analgesics, antibiotics, antiepileptic agents, asthma and allergy agents, vaccines and insulin products[6]. Dosing errors among studies conducted in US ranged between 15% to 34%. The incidences of dose error per 100 medication orders was 38.9[7]. Dosing errors habitually associated in the harmful or hypothetically harmful errors, mainly during the prescribing stage. The incidence of medication errors in UK hospitals are virtually similar to those in the USA, in which prescribing errors occur in 1.5% of the prescriptions while 3 to 8% of doses given has administration errors[8, 9]. Incidence of medical errors in a tertiary care pediatric unit in India was 35.5% and severe morbidity due to errors was seen in 2.4%[10].
Commencing a number of pharmacoepidemiological studies in pediatrics well recognized that the risk of ADRs increases with the span of the patient's stay in hospital, the number of medicines she or he is accepting, the level of off-label use and the dynamics of physiological changes through early life. Moreover, retrospective and prospective monitoring from computerized medical records requiring a more or less passive role of the health professionals and it is time effective compared with other intensive surveillance systems. It will probably improve spontaneous reporting of pharmacological, unpredictable and not dose-related reactions and in part preventable, dose-related reactions to a medicine[11]. So, the present study was undertaken to evaluate the medication safety in children’s
Materials and Methods
A prospective observational study was conducted in the PICU, NICU, IP and OP department of Paediatrics at Rajiv Gandhi Institute of Medical Sciences overa 6-monthsperiod(from august 2012 toJanuary 2013). The Institutional Ethics and Research Committee of Rajiv Gandhi Institute of Medical Sciences, Kadapa (Rc.No.413/Acad./2011-12), approved the study. All patients under 12 year of age were included in the study. Medications, which are not available in RIMS drug formulary. Patients referred from another health facility or hospital to collect their medications from the pharmacy of the respective health clinics were excluded from the study. A standardized data collection form was designed for the purpose of this study. All documented medication orders monitored for any drug misadventures and recommended to the pediatricians.
Results
Drug misadventures include medication errors 588 (84%), ADEs 27 (3.8%), ADRs 33 (4.8%) and drug interactions 52 (7.4%).
Medication errors
The total number of medication errors detected was 588 (84%) with 29.9% in NICU, 18.1% in PICU, 14.4% in pediatrics inpatient department and 37.6% in pediatrics out patient department. Majority errors were found in the age group of 1-3 years. Month wise medication errors were expressed in table 1.Types of errors were expressed in figure 1. Physiological system wise medication errors were expressed in table 2 and medicine class wise expressed in figure 2. Severity level of medication errors were expressed in table 3.Psychology of medication errors included mistakes 286 (65%) and slips 106 (35%) is expressed in figure 3.Strategies to reduce medication errors included reduce in prescribing errors 275 (46.76%), reduce in calculation errors 172 (29.25%) and providing patient information 141 (23.97%) is expressed in table 4.We observed majority of medication error is inappropriate dose error. Therefore, we find out the incidence, nature of dosing errors and problems in scaling adult drug doses to children. Incidence of dosing errors was 35.28%. Nature of dosing errors expressed in figure 4 and Problems in scaling adult drug doses to children were expressed in table 5.
Adverse drug events
The total number of adverse drug events noticed was 27 (3.8%) with 0.8% in NICU, 0.5% in PICU, 1.7% in pediatrics inpatient department and 0.8% in pediatrics out patient department.Majority errors were found in the age group of 1-3 years. Physiological system wise adverse drug events were expressed in table 6 and Therapeutic group of the drugs associated with adverse drug events was expressed in table 7. Classification of adverse drug events was expressed in table 8.
Adverse drug reactions
The total number of adverse drug reactions perceived was 33 (4.8%) with 1.4% in NICU, 0.9% in PICU, 1.6% in pediatrics inpatient department and 0.9% in pediatrics out patient department.Majority of adverse drug reactions were found in the age group of 1-3 years. Causality assessment of adverse drug reactions was expressed in table 9.Therapeutic group of the drugs associated with the adverse drug reactions is shown in table 10.Physiological system wise adverse drug reactions were expressed in table 11. Mechanism of adverse drug reactions was expressed in table 12.
Drug interactions
The total number of adverse drug reactions perceived was 52 (7.4%). Majority of drug interactions were found in the age group of 4-6 years. Severity level of drug interactions was expressed in table 13.Types of drug interactions include drug-drug interactions 3%, and drug-food interactions1.4% and drug nutrient interactions0.2%.
Table No.1: Month wise medication errors (n=588)
|
Month |
No. of medication orders |
% |
|
August |
134 |
22.6 |
|
September |
118 |
20.0 |
|
October |
103 |
18.1 |
|
November |
91 |
16.5 |
|
December |
78 |
12.0 |
|
January |
64 |
10.8 |
Table No.2: Physiological system wise medication errors (n=588)
|
Physiological system |
No. of medication orders |
% |
|
Cardiovascular system |
2 |
0.3 |
|
Respiratory system |
116 |
19.8 |
|
Central nervous system |
57 |
9.7 |
|
Gastrointestinal system |
122 |
20.8 |
|
Endocrine system |
1 |
0.2 |
|
Genitourinary system |
47 |
8.0 |
|
Nutrition & Blood |
35 |
5.9 |
|
Infections |
157 |
26.7 |
|
Others |
47 |
8.0 |
Table No. 3: Severity level of medication errors (n=588)
|
Level of severity |
No. of medication orders |
% |
|
|
No Error |
Category-A |
58 |
9.8 |
|
Error, No harm |
Category-B |
144 |
24.5 |
|
Category-C |
196 |
33.3 |
|
|
Category-D |
113 |
19.2 |
|
|
Error, Harm |
Category-E |
44 |
7.5 |
|
Category-F |
33 |
5.6 |
|
|
Category-G |
0 |
0 |
|
|
Category-H |
0 |
0 |
|
|
Error, Death |
Category-I |
0 |
0 |
Table No.4: Strategies to reduce medication errors (n=588)
|
Strategies |
No. of medication orders |
% |
|
Providing Patient information |
141 |
23.97 |
|
Reduce calculation errors |
172 |
29.25 |
|
Reduce prescribing errors |
275 |
46.76 |
Table No.5: Problems in scaling adult drug doses to children (n=700)
|
Problem |
No. of medication orders |
% |
|
Age and weight |
446 |
63.7 |
|
Disease |
219 |
31.3 |
|
Pharmaceutical formulation |
35 |
5.0 |
Table No.6: Physiological system wise adverse drug events (n=27)
|
Physiological system |
No. of medication orders |
% |
|
Cardiovascular system |
1 |
0.1 |
|
Respiratory system |
9 |
1.3 |
|
Central nervous system |
3 |
0.4 |
|
Gastrointestinal system |
14 |
2.0 |
Table No.7: Therapeutic group of the drugs associated with the adverse drug events (n=27)
|
Therapeutic group |
No. of medication orders |
% |
|
Antihistamines |
1 |
0.1 |
|
Anti-emetic’s |
9 |
1.3 |
|
Respiratory system agents |
3 |
0.4 |
|
Antimicrobial agents |
14 |
2.0 |
Table No.8: Severity level of adverse drug events (n=27)
|
Severity |
No. of medication orders |
% |
|
Severe |
3 |
0.4 |
|
Mild |
14 |
2.0 |
|
Moderate |
9 |
1.3 |
|
Lethal |
1 |
0.1 |
Table No.9: Causality assessment of adverse drug reactions (n=33)
|
Causality |
No. of adverse drug reactions |
% |
|
Probable |
13 |
1.8 |
|
Possible |
19 |
2.7 |
|
Definite |
1 |
0.1 |
|
Unlikely |
0 |
0 |
Table No.10: Therapeutic group of the drugs associated with the adverse drug reactions (n=33)
|
Therapeutic group |
No. of medication orders |
% |
|
Antihistamines |
1 |
0.1 |
|
Antimicrobial agents |
8 |
1.1 |
|
Respiratory system agents |
9 |
1.3 |
|
Analgesics and antipyretics |
15 |
2.1 |
Table No.11: Physiological system wise adverse drug reactions (n=33)
|
Physiological system |
No. of medication orders |
% |
|
Cardiovascular system |
1 |
0.1 |
|
Respiratory system |
11 |
1.5 |
|
Central nervous system |
4 |
0.4 |
|
Gastrointestinal system |
17 |
2.4 |
Table No.12: Mechanism of adverse drug reactions (n=33)
|
Mechanism |
No. of medication orders |
% |
|
Idiosyncrasy |
1 |
0.1 |
|
Hypersensitivity |
17 |
2.4 |
|
Intolerance |
11 |
1.5 |
|
Pharmacologic |
4 |
0.4 |
Table No.13: Severity of drug interactions (n=52)
|
Severity |
No. of medication orders |
% |
|
Minor |
17 |
2.4 |
|
Moderate |
15 |
2.1 |
|
Major |
1 |
0.1 |

Figure 1: Types of medication errors (n=588)
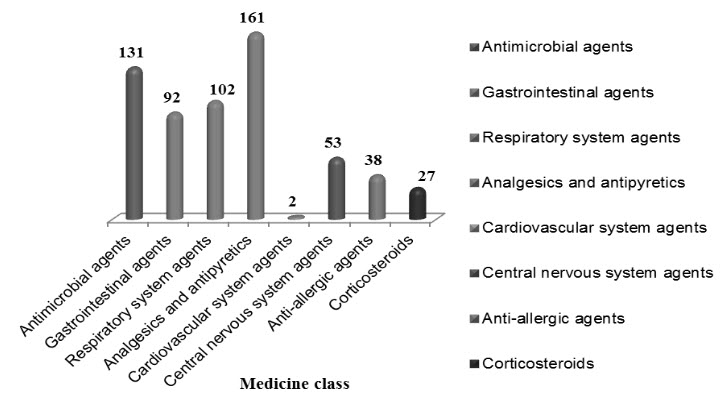
Figure 2: Medicine class wise medication errors (n=588)
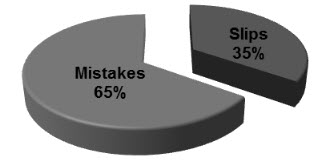
Figure 3: Psychology of medication errors (n=588)

Figure 4: Nature of dosing errors (n=700)
Discussion
Pediatric inhabitants by itself are a range of diverse physiologies with substantial disparity in pharmacokinetics and pharmacodynamics. Tactlessly, 50–90% of drugs used in children today never been essentially studied in this population and the consequences of drug studies done in adults are frequently extrapolated for use in children. Many drugs used in children are unlicensed, off-label, unsafely or without any confirmation of efficacy in children. A report of WHO in 2005 showed three times more medical errors in children than in adults.Result show that drug misadventures in children were common. The variables that show the most were medication errors, adverse drug reactions and drug interactions.Majority medication errors were found in the pediatrics O.P (37.6%). In our study, the most communal type of prescribing error (35.28%) was related to an incorrect dose (either too high or too low). In one study accompanied by Lesar et al. [12]establish that the most common type of prescribing error (56.1%) was related to an incorrect dose (either too high or too low).Majority of errors met in analgesics and antipyretics (23%) tracked by anti-microbial agents (18.7%) because of their regular usage. A Comparable study conducted by RainuKaushal[13]proved that most medication errors found in anti- infective drugs (28%). Intensity of workload was predictable to be risk factor for medication errors. Severity level assessment of medication errors revealed that majority of errors (64.7%) were dropped under the category-B, C and D (Error, No Harm). 9.8% were in the category-A (No Error) and 13.1% in the category-E, F (Error, Harm). A parallel study was conducted by Marcin JP et al [14]revealed that among the 69 patients with medication errors, 11 had errors categorized as impending to cause harm (15.9%) and 58 had errors categorized as causing no harm (85.5%). The physician psychology is a part in medication errors, which shows that 65% were mistakes and 35% were slips.Prescriber’s workload, lack of sufficient knowledge and time spent with one patient are restrains that lead to most of the mistakes. Recognition of these errors is the first step in their prevention. The information available shows that medication errors (dosing errors) are common and most of them prescribed are 10-fold higher than relative daily dose, have led to serious concerns. There is a crucial need to develop methods to reduce medication errors in children should be the major priority. Therefore, strategies to reduce medication errors shows that 46.76% were reducing in prescribing followed by 29.25% were reducing in calculation errors. It compounded by the fact that prescriptions often written by the most junior doctors who may be unfamiliar with the medicine.Our study identified higher dosing errors (35.28%) when compared to dosing errors among studies conducted in US is ranged between 15% to 34%. Eleven of the 16 studies determined that dosing errors are the most common type of medication error, three of the remaining studies determined that it is the second most common type.[15] Nature of dosing errors shows that 64.7 % prescriptions were with normal dose, 20.5% with over dose and 14.7 % with under dose. Problems in scaling adult drug doses to children show that age and weight accounts for 63.7 %, followed by disease 31.3% and pharmaceutical formulation 5%.
The statement in this frame work was that reducing error would translate directly into reducing harm. Majority adverse drug events noticed was 27 (3.8%) with 1.7% in paediatrics inpatient department followed by 0.8% in pediatrics out patient department. Majority errors were found in the age group of 1-3 years. Physiological system wise adverse drug events stated that 2% accounts for gastrointestinal system followed by 1.3% respiratory system. Therapeutic group of the drugs associated with adverse drug events revealed that 2% antimicrobials, followed by 1.3% anti emetics. Classification of adverse drug events stated that mild 2%, followed by 1.3% moderate. In one study, Kaushal and colleagues[16]reported ADE rates in children on inpatient wards at two urban teaching hospitals to be 2.3 per 100 admissions (26 events), with an additional potential ADE rate of 10 per 100 admissions (115 events).In the second study, Holdsworth and coworkers [17]reported an ADE rate in pediatric inpatients(pediatric ICU and general care unit at a university hospital) of six per 100 admissions (76 events), with 61% classified as preventable, and a potential ADE rate of 8 per 100 patient days (94 events). Woods and colleagues, [18]by means of retrospective chart reviewof 3719 randomly selected discharges of patients between 0 and 20 years old hospitalized in Utah and Colorado, report AE rates of one per100 patients (39 events), with 59% classified as preventable. Extreme less research and information are available concerning the incidence of AEs and ADEs in outpatient settings.Gurwitz and colleagues [19]report an ADE rate of 50.1 per 1000 person-years in a large adult outpatient population, with 38% characterized as serious, life threatening, or fatal. In this study, the most common drug classes associated with outpatient ADEs were cardiovascular medications(24.5%), diuretics (22.1%), and non-opioid analgesics (15.4%).[20, 21]Similar to research of inpatient settings, the majority of preliminary research efforts concentrated on medication errors rather than ADEs.In pediatrics, few studies assess error or harm in outpatient settings.A current study of a pediatric population by McPhillips and colleagues[22]attentive on medication errors in an outpatient setting and shown 15% of all prescriptions dispensed contained a medication error; 8% of these prescriptions reflected an overdose and 7% reflected an underdose.In this study, children between 0 and 3 years of age, and children who had six or more medications dispensed are at particular risk. The medication classes utmost at risk for errors included antiepileptic medications (errors 21%of the time), asthma and allergy medications (errors 19% of the time), and analgesics (errors 16% of the time). Causality assessment of adverse drug reactions revealed that majority was probable 2.7%, followed by possible 1.8%. Physiological system wise adverse drug reactions stated that 2.4% accounts for gastrointestinal system followed by 1.3% respiratory system. Therapeutic group of the drugs associated with adverse drug reactions revealed that 2.1%analgesics and antipyretics, followed by 1.3% respiratory system agents. Mechanism of adverse drug reactions revealed that majority was hypersensitivity 2.4%, followed by intolerance 1.5%. Severity level of drug interactions revealed that minor 2.4%, followed by moderate 2.1%. The limitation of this study is identification of less number of drug misadventures because of short period.
Conclusion
It can be concluded that drug misadventures are common in children. Moreover, retrospective and prospective monitoring from computerized medical records requiring a more or less passive role of the health professionals and it is time effective Compared with other intensive surveillance systems. It will probably improve spontaneous reporting of pharmacological, unpredictable and not dose-related reactions and in part preventable, dose-related reactions to a medicine.
Acknowledgements
We owe our whole hearted thanks to Principal Dr. K. Ravindra Reddy, whose constant motivation and all the timely help throughout our project work.
References
1.Leelavathi D. Acharya, JavedShareef, Padma G. M. Rao., Study and Evaluation of Medication Errors in a multidisciplinary tertiary Care South Indian Teaching Hospital.Indian Journal of Hospital Pharmacy.2008; 45: 54-58.
2.The importance of pharmacovigilance: safety monitoring of medicinal products. Geneva, World Health Organization, 2002.
3.Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16): 2114-2120.
4.Ross LM, Wallace J, Paton JY. Medication errors in a pediatric teaching hospital in the UK: Five years operational experience.Arch Dis Child. 2000; 83: 492-7,.
5.Bronstein AC, et al. 2010 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th annual report. ClinlToxicol, 49, 910-9412011.
6.Centers for Disease Control and Prevention. CDC WONDER Compressed Mortality File, Underlying Cause-of-Death. Centers for Disease Control and Prevention Website.Available from: wonder.cdc.gov/mortSQL.html.Accessed on: February 15, 2012.
7.McPhillips HA, Stille CJ, Smith D, et al. Potential medication dosing errors in outpatient pediatrics.JPediatr. 2005; 147: 761-7.
8.Wong IC, Ghaleb MA, Franklin BD, et al. Incidence and nature of dosing errors in paediatric medications: a systematic review. Drug Saf.2004; 27(9):661–670.
9.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in paediatric inpatients. JAMA. 2001;285(16):2114–2120.
10.Mansiparihar and Gouriraopassi.Medical errors in pediatric practice.Indian pediatrics.2008; 45: 586-89.
11.Conroy S, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. British Medical Journal.2000; 320: 79-82.
12.Lesar TS, Lomaestro BM, Pohl H. Medi
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 3 Received On: 11/01/2014; Accepted On: 31/01/2014; Published On: 05/03/2014 How to cite this article: L Reddenna, Medication Safety in Children, PharmaTutor, 2014, 2(3), 124-133 |









