About Author:
Vivek P. Chavda
Department of Pharmaceutics, B.K. Mody Government Pharmacy College,
Rajkot – 360003, Gujarat (India)
vivek7chavda@gmail.com
Introduction
A number of countries require manufacturers of industrial chemicals, pharmaceuticals, veterinary drugs, pesticides, cosmetic products, food products, feed additives, etc., to establish through data that use of these products do not pose any hazards to human health and the environment. Non-hazardous nature needs to be established through studies and data, which will be examined by the regulatory authorities of the concerned countries. Good Laboratory Practice (GLP) is a system, which has been evolved by Organisation for Economic Co-operation and Development (OECD) used for achieving the above goals. National GLP Compliance Monitoring Authority was established by the Departmentof Science & Technology, Government of India, with the approval of the Union Cabinet on April 24, 2002. GLP-compliance certification is voluntary in nature. Industries/ test/ facilities/ laboratories dealing with above chemicals and looking for approval from regulatory authorities before marketing them, may apply to the National GLP Compliance Monitoring Authority for obtaining GLP Certification.[1] “GLP embodies a set of principles that provides a framework within which laboratory studies are planned performed, monitored, reported and archived.”
REFERENCE ID: PHARMATUTOR-ART-1933
History[2-5]
GLP was first introduced in New Zealand and Denmark in 1972. GLP was instituted in US following cases of fraud generated by toxicology labs in data submitted to the FDA by pharmaceutical companies. Industrial BioTest Labs(IBT) was the most notable case, where thousands of safety tests for chemical manufacturers were falsely claimed to have been performed or were so poor that police investigators could not piece together what work had been done. even though IBT superficially delivered the test results their contracts with the manufacturers specified. These issues were made public in the hearings at the US Congress, which led to the FDA’s publication of Proposed Regulations on GLP in 1976, with establishment of the Final Rule in June 1979 (21 CFR 58). The Environmental Protection Agency (EPA) had also encountered similar problems in submitted to it, and issued its own draft GLP regulations in 1979 and 1980, publishing the Final Rules in two separate parts (40 CFR 160 and 40 CFR 792) in 1983. In 1981 an organization named OECD produced GLP principles that are international standard.
Why GLP?[6]
In the early 70’s FDA became aware of cases of poor laboratory practice all over the United States. FDA decided to do an in-depth investigation on 40 toxicology labs. They discovered a lot fraudulent activities and a lot of poor lab practices. Examples of some of these poor lab practices found were:
1. Equipment not been calibrated to standard form, therefore giving wrong measurements.
2. Incorrect/inaccurate accounts of the actual lab study
3. Inadequate test systems
Objectives of GLP[7]
1. GLP makes sure that the data submitted are a true reflection of the results that are obtained during the study.
2. GLP also makes sure that data is traceable.
3. Promotes international acceptance of tests.
Principal of GLP
GLP principles include:
1. Organization and Personnel
Management-Responsibilities
Sponsor-Responsibilities
Study Director-Responsibilities
Principal Investigator-Responsibilities
Study Personnel-Responsibilities
2. Quality assurance program
Quality Assurance Personnel
3. Facilities
Test System Facilities
Facilities for Test and Reference Items
4. Equipment, reagents and Materials
5. Test systems
Physical/Chemical
Biological
6. Test & Reference items
7. Standard operating procedures
8. Performance of Study
Study Plan
Conduct of Study
9. Reporting of results
10. Storage of Records and Reports
OECD publishes OECD Guidelines for the Testing of Chemicals, which are guidelines that usually have to be followed for GLP compliance. They are widely required by agencies doing risk assessments of chemicals.The United States FDA has rules for GLP in 21CFR58. Preclinical trials on animals in the United States of America use these rules prior to clinical research in humans.Research in the US not conducted under these restrictions or research done outside US not conducted according to the OECD Guidelines (or FDA rules) might be inadmissible in support of a New Drug Application in the US.[7]
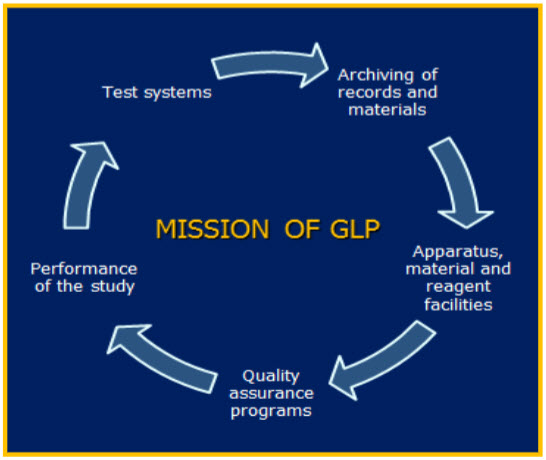
Figure 1: Mission of GLP
GLP Essentials[8]
a. The instructions and procedures are written in clear English or local languages.
b. Operators are trained to carry out procedures correctly.
c. Records are made to during laboratory works.
d. All laboratory processes are clearly defined, systematically reviewed in the light experiences.
e. All necessary facilities are provided including:
· Approx qualified and trained personnel.
· Adequate premises and Space.
· Suitable equipments and services.
· Correct containers, materials and labels
· Approved procedures and instructions.
· Suitable storage and transport.
· Adequate personnel, equipment for all type of in process tests and management under the responsibility of the management.
f. The proper storage and distribution of the productsminimizes any risks of their quality.
Utility and importance of GLP
GLP is obviously so crucial to modern laboratory operations, but most importantly because good laboratory practice is an essential ingredient for any professional scientist, this course will incorporate many of the principles that are part of GLP in contemporary laboratories.It is not appropriate to implement a full complement of GLP policies for a student laboratory, as the experimental studies are not related to a commercial product. However, it is useful to incorporate those GLP policies which are fundamental to any sound laboratory work, and to provide an introduction to GLP policies that are a part of any contemporary commercial laboratory.
GLP is needed for;
- Non clinical safety studies
- Agricultural pesticide development
- Development of toxic chemicals
- Food control
- Test of substances with regard to explosive hazards
GLP is not needed for;
- Basic research
- New analytical method development
- Chemical tests
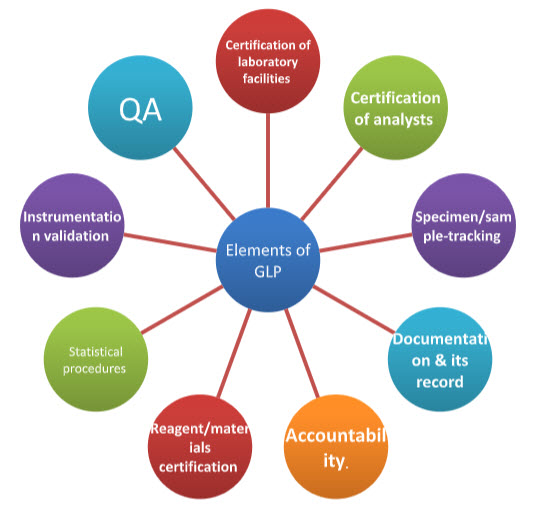
Figure 2: Elements of GLP
1. Laboratory Infrastructure[9]
Whatever the industry targeted, GLP stresses the importance of the following main points:
1. Resources: Organisation, personnel, facilities and equipment;
2. Characterisation: Test items and test systems;
3. Rules: Protocols, standard operating procedures (SOPs);
4. Results: Raw data, final report and archives;
5. Quality Assurance: Independent monitoring of research processes.
Modern Quality Control Laboratory should have followingsections;
A. General Chemical Laboratory
It should be wellventilated with good construction. Preferably it should be air conditioned tomaintain a temperature of 27°C± 1° C.
B. Instrument Room
The temperature should be 25°C± 1°C and RH of 45± 5% in this area. It should have a centrallyregulated constant supply voltage of 230 volts ± 1% and afrequency of 27Hz± 3%. There should be separate room forhousing semi-micro and microbalances.
C. Microbiological Laboratory
The laboratory must be airconditioned, preferably with an AHU suitable filter (5micron or less), For units having both sterile and nonsterileproducts there should be two aseptic zones having class1,000 area with LAF and entry through graded air zones,one for inoculation and culture transfer and one for sterilitytesting. For units having only non-sterile products, oneaseptic zone must be there.
D. Hot zone
Ventilationis a key element in such area and it consists of antoclaves, hot air oven, mufflefurnance, furne cupboard etc.
E. Package Material Testing Section
It should haveadequate space to accommodate instruments with appropriate furniture to store the supplementary materials.
F. Retained Sample Area
Proper temperature control ismust in this area and this should be provided for storage andpreservation.
G. Cleaning Area
Suitable facilities like running hot &cold water, purified water, different cleaning agents etc.should be essential for this area.
H. Storage area for laboratory chemicals, glass apparatusand miscellaneous items
Proper temperature control isrequired for this area. The cupboard used for the storage should be air tight and resistance to corrosion.
I. Others
The suitable arrangements are required for Q.C.lab in different section such as vaccum, compressed air,nitrogen, potable water, purified water, ultra-pure water etc.
2. Reference Standard and Reference MicrobialCultures [9]
The primary reference standards for active and inactive bulkdrugs are to be procured periodically and these standardsare to be preserved properly (i.e. temp, RH etc).Reference microbial cultures are to be procured from thestandard institutions whenever required. These cultures areto be maintained properly in the microbial lab as perinstructions from the suppliers or as per pharmacopoeia.Proper documentation also required for this case.
3. Use of Analytical Reagents and Chemicals
All the chemicals utilized should be of analytical grade at least. Documentation is crucial for all of them. It is desirable if such reagents are given proper code to identify them.
4. Use of Volumetric Glasswares
The all volumetric glasswares should be of two gradeswhich are used in the laboratory. Class A are to be used forwork of the highest accuracy like standardization ofvolumetric solutions and class B for routine work.
5. Preparation of Standard Solutions and Reagent [9]
All standard solutions, reagents must have proper labelsindicating name, strength, date of preparation, date ofexpiry and storage conditions.
6. Calibration of Equipment’s and Instruments [11]
For the proper conduct of the study, appropriate equipment of adequate capacity must be available. All equipment should be suitable for its intended use, and it should be properly calibrated and maintained to ensure reliable and accurate performance. Records of repairs and routine maintenance and of any non-routine work should be retained. Remember that the purpose of these GLP requirements is to ensure the reliability of data generated and to ensure that data are not invalidated or lost as a result of inaccurate, inadequate or faulty equipment.Calibration is the comparison of the performance of ameasuring equipment/instrument with that of standardequipment/instrument. It may be divided into threecategories:
a. Calibration by external agency: e.g. Pressure gauge,thermo dials, glass thermometers, wet and dry bulbhygrometers, balances etc.
b. Calibration in the laboratory: e.g. UV VisSpectrophotometer, Polarimeter etc.
c. Calibration in the laboratory with the help of externalagency: e.g. HPLC, particle counters etc.
Maintenance may be carried out in two quite distinct ways:
* Preventive maintenance;when parts are changed regularly based upon the expected life of the part concerned. Planned maintenance of this type may be a useful precaution for large items of equipment or items that do not possess suitable backup or alternatives. Regular preventive maintenance therefore reduces the risk of breakdown.
* Curative maintenance;when repairs are made in the case of a fault being detected. This approach particularly applies to equipment such as modern computer driven analysers or electronic balances that do not easily lend themselves to preventive maintenance. It is good practice to adopt contingency plans in case of failure; these may include having equipment duplicated or assuring that there is immediate access to a maintenance technician or an engineer.
7. Validation of Analytical Procedure
The various aspects are accuracy, precision, specificity,linerity and range, limit of detection, limit of quantitation,robustness, ruggedness etc.
8. Training [11]
All laboratory personnel i.e. managers, supervisory staff,analyst, technical helpers etc. should have regular training. There is certain newer technological advancement commenced with respect to instrumentation for which updation ok knowledge is required. Training may be two types. The formal training of any new worker includes analyticalchemistry, statistical techniques, microbial techniques,instrumental techniques, electronic data processing,documentation etc. The second type namely informalrecords of training.The training system should have elements common to all GLP management systems,i.e. it is:
• Formal.
• Approved.
• Documented to a standard format.
• Described in a Standard Operating Procedure.
• Possible to perform an historical reconstruction of training through the archived documents.
9. Documentation and Records[10]
The Study Director uses the records as the basis for the scientific interpretation of the study. This interpretation, as well as an accurate representation of the data, will be incorporated into the final report of the study. The authorship of the final report is the responsibility of the Study Director. Finally, at study completion, all the documents, both prescriptive and descriptive, are archived so that when necessary full study reconstruction will be possible through examining the archived material.The following documents and records are included:
- Specification
- Test procedure
- Standard operation
- Certificate of Analysis with relevant test protocols
- Sample Register.
- Register for records.
- Registered for retained samples (Both finishedproducts and active raw materials).
- Records pertaining to the preparation of solutions ofreference standards, volumetric solutions and otherreagents.
- Log book instruments and equipments.
There are also specific elements required by GLP which must be in the study report:
• Name and address of test facility.
• Dates of start and finish of experimental work.
• Name of Study Director.
• Study objectives.
• Details of test item(s) and vehicle(s).
• Description of test system.
• Details of dosing, route and duration.
• Results.
• Statistics.
• Discussion.
• References.
• GLP Compliance Statement from Study Director.
• QA statement of Inspections/Audits
• Signed/dated reports from responsible scientists.
10. Safety [9]
The uses of mask, gloves, face shields, aprons, gumbootsetc. should be compulsory in the handling of corrosivechemicals. There should be adequate firefightingarrangements in the laboratory and personnel should begiven proper training for firefighting.
GLP and Automated Systems[5, 11]
In many instances, the optimal recommended 'no-argument' means of implementing GLP is to develop an automated approach to both Sample Preparation and Sample Measurement. If this can include an overarching 'chain of custody' sample history and data flow, combined with adequate SOP's for calibration & linearization of measuring tools, GLP compliance is virtually assured.Implementing GLP on an automated system, as an intellectual and labour-intensive task, requires a GxP company to make a great amount of effort. To ease the burden of this management, Webster et al. have provided a tutorial for users to quickly embark on and do the job properly. Certain Manufacturers & Vendors of Laboratory Instrumentation and/or Laboratory Automation are well-versed in assisting with the application of GLP (and 21CFR11) to their commercial offerings.
Grounds for Disqualification
1. The testing facility failed to comply with one or more regulations implemented by the GLP manual
2. The failure to comply led to adverse outcomes in the data; in other words, it affected the validity of the study
3. Warnings or rejection of previous studies have not been adequate to improve the facility’s compliance
Consequences of Noncompliance
The FDA states the following consequences of noncompliance:
1. The commissioner will send a written proposal of disqualification to the testing facility
2. A regulatory hearing on the disqualification will be scheduled
3. If the commissioner finds that after the hearing, the facility has complied, then a written statement with an explanation of termination of disqualification will be sent to the facility
Thus, if it can be shown that such disqualifications did not affect the integrity and outcome of the study itself, or did not occur at all, then the study may be reinstated at the will of the commissioner
References
[1] Government Document. National good laboratory practice. DST 2013:7.
[2] Robinson K. GLPs and the Importance of Standard Operating Procedures. BioPharm International Aug 1, 2003:7.
[3] Schneider K. Faking it: The case against Industrial Bio-Test Laboratories. Amicus Journal 1983;Spring:14–26.
[4] Staff WHO. Handbook: Good Laboratory Practice (GLP). World Health Organization 2013:328.
[5] Wikipedia. http://en.wikipedia.org/wiki/Good_Laboratory_Practice. Accessed on 7th July,2013.
[6] Gladney L, Osakwe I, Ford E. Good Laboratory Practices. Powerpoint presentation 2013:30 slides.
[7] ENV/MC/CHEM(98) 17. OECD Principles of Good Laboratory Practice. OECD, 1998;Paris,.
[8] Garner WY, Barge MS. Good Laboratory Practice: An Agrochemical Perspective. ACS Symposium Series 1989:369.
[9] Saha D, Jain V. Good Laboratory Practice: Design and Utility. Asian J Pharm Tech 2011;1:01-3.
[10] WHO. Quality assurance of pharmaceuticals : A compendium of guidelines and related materials. Good manufacturing practices and inspection 1999;WHO Geneva: Volume 2.
[11] Kott L, Maloney T. JALA Tutorial: Considerations When Implementing Automated Methods into GxP Laboratories. Journal of the Association for Laboratory Automation 2005;10:182–91.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









