{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
L Reddenna1*, Dr. P. Venkatesh1, K Siva Kumar2, A Sai keshava Reddy2
1* Department of Pharmacy Practice,
Jagan’s College of Pharmacy,
Nellore, Andhra Pradesh, India
2 Department of Pharmacy Practice,
Nirmala College of Pharmacy,
Kadapa, Andhra Pradesh, India
*reddennapharmd@gmail.com
ABSTRACT
Genotoxicity describes the possessions of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. Heritable changes can influence either somatic cells of the organism or germ cells to be passed on to future generations. As a result, many urbane techniques including Ames Assay, in vitro and in vivo Toxicology Tests, and Comet Assay have been developed to evaluate the chemicals probable to cause DNA damage that may lead to cancer. The genotoxic substances provoke damage to the genetic material in the cells through exchanges with the DNA sequence and structure. Genotoxicity testing is to resolve if a substrate will sway genetic material or may cause cancer. Genotoxic Chemotherapy is the treatment of cancer with the use of one or more genotoxic drugs. The treatment is traditionally part of standardized regime. By utilizing the destructive properties of genotoxins treatments aims to induce DNA damage into cancer cells.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2523
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 9 Received On: 13/04/2017; Accepted On: 22/05/2017; Published On: 01/09/2017 How to cite this article: Reddenna L, Venkatesh P, Kumar KS, Reddy ASK;Genotoxicity; PharmaTutor; 2017; 5(9);23-34 |
INTRODUCTION
Genotoxicity describes the possessions of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. Genotoxicity is perplexed with mutagenicity; all mutagens are genotoxic, while not all genotoxic substances are mutagenic. The variation can have direct or indirect effects on the DNA
• The induction of mutations
• Mistimed event activation
• Direct DNA damage leading to mutations.
Genetic Toxicology
• Study of an agent’s ability to induce genetic damage
• Subspecialty of toxicology
• Current paradigm developed over last 30 years
• Short-term in vitroand in vivogenetic toxicology tests [1]
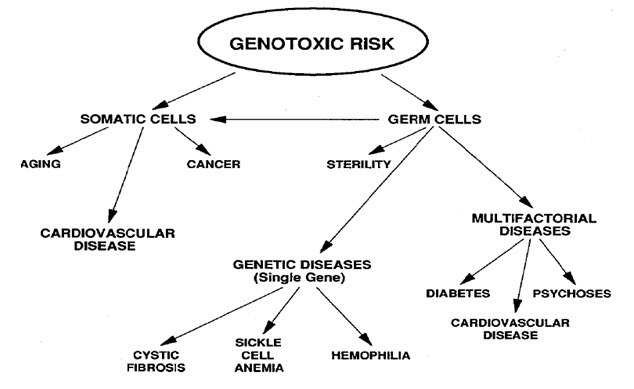
Figure-1: It displays the genotoxic risk
Heritable changes can influence either somatic cells of the organism or germ cells to be passed on to future generations. Cells avert expression of the genotoxic mutation by either DNA repair or apoptosis; Conversely, the smash up may not always be preset leading to mutagenesis. To assay for genotoxic molecules, DNA damage in cells exposed to the toxic substrates. This DNA damage can be in the type of single and double strand breaks, thrashing of excision repair, cross-linking, alkali labile sites, point mutations, and structural and numerical chromosomal aberrations. The compromised veracity of the genetic material has been known to cause cancer. As a result, many urbane techniques including Ames Assay, in vitro and in vivo Toxicology Tests, and Comet Assay have been developed to evaluate the chemicals probable to cause DNA damage that may lead to cancer.
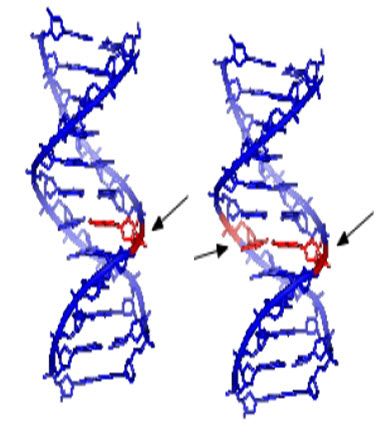
Figure-2: Single and double strand DNA damage potentially caused by genotoxins
Mechanisms
The genotoxic substances provoke damage to the genetic material in the cells through exchanges with the DNA sequence and structure. Example, the transition metal chromium interacts with DNA in its high valent oxidation state so to incur DNA lesions leading to carcinogenesis. A research performed to study the interface between DNA with the carcinogenic chromium by using a Cr (V) Salen complex at the specific oxidation state. The interface was explicit to the guanine nucleotide in the genetic sequence. High-valent chromium is seen to act as a carcinogen; found that the mechanism of damage and base oxidation products for the interaction between high-valent chromium and DNA are applicable to in vivo formation of DNA damage leading to cancer.

Figure-2: Transitions and transversions
An additional example of a genotoxic substance causing DNA damage is pyrrolizidine alkaloids (PAs). These substances are found mostly in plant species and are poisonous to animals, including humans; About half of them have been acknowledged as genotoxic and many as tumorigenic. The pyrrolizidine alkaloids are mutagenic in vivo and in vitro and, therefore, accountable for the carcinogenesis outstandingly in the liver. [2]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Techniques
Genotoxicity testing is to resolve if a substrate will sway genetic material or may cause cancer. They can be performed in bacterial, yeast, and mammalian cells. With the information from the tests, one can manage early development of vulnerable organisms to genotoxic substances.
Ames test
Ames Assay is used in laboratories to check for gene mutation. The technique uses many diverse bacterial strains in order to evaluate the different changes in the genetic material. The result of the test detects the mainstream of genotoxic carcinogens and genetic changes; the types of mutations detected are frame shifts and base substitutions.
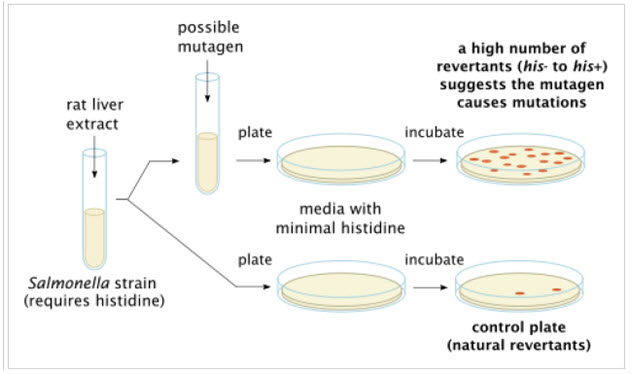
Figure-3: Ames test procedure for gene mutations present in various bacterial strains
Bacterial Mutation Assay
* DNA Damage Detected
- Base pair substitutions (-ATGC-ACGC)
- Frameshiftmutations (-ATGC-ATTGC)
* Ames or Salmonella Assay
- Developed by Bruce Ames early 1970s
- Most widely used, fast, easy and cost-effective
* 5 Bacterial strains engineered to be more sensitive to damage (rfa, uvrB-, pKM101)
- Alteration to cell wall, increased permeability
- Removal of excision repair & introduction of error-prone repair

Figure-4: It displays the bacterial mutation assay
The principle of in vitro testing is to conclude whether a substrate, product, or environmental factor induces genetic damage. Cytogenetic assays using different mammalian cells. The types of aberrations detected in cells pretentious by a genotoxic substance are chromatid and chromosome gaps, chromosome breaks, chromatid deletions, fragmentation, translocation, complex rearrangements, and many more. The clastogenic or aneugenic effects from the genotoxic damage will cause an increase in frequency of structural or numerical aberrations of the genetic material. Gene mutations are commonly point mutations, altering only one base within the genetic sequence to alter the resultant transcript and amino acid sequence; These point mutations comprise base substitutions, deletions, frameshifts, and rearrangements. Chromosomes' integrity may be distorted through chromosome loss and clastogenic lesions causing numerous gene and multilocus deletions. The exact type of damage is determined by the size of the colonies, distinctive between genetic mutations (mutagens) and chromosomal aberrations (clastogens).
Umu assay
The umu assay test evaluates the capability of a substance to induce DNA damage; it is based on the alterations in the induction of the SOS response due to DNA damage. The benefits of this technique are that it is a fast and simple method and suitable for several substances. These techniques are performed on water and wastewater in the environment.

In vivo Testing
The purpose for in vivo testing is to determine the potential of DNA damage that can affect chromosomal structure or disturb the mitotic apparatus that changes chromosome number; the factors that could influence the genotoxicity are ADME and DNA repair. It can also detect genotoxic agents missed in in vitro tests. The positive resultof induced chromosomal damage is an increase in frequency of micronucelated PCEs. A micronucleus is a small structure separate from the nucleus containing nuclear DNA arisen from DNA fragments or whole chromosomes that were not incorporated in the daughter cell during mitosis. Causes for this structure are mitotic loss of acentric chromosomal fragments (clastogenicity), mechanical problems from chromosomal breakage and exchange, mitotic loss of chromosomes (aneugenicity), and apoptosis. The micronucleus test in vivo is similar to the in vitro one because it tests for structural and numerical chromosomal aberrations in mammalian cells, especially in rats' blood cells. [3]
Comet Assay
Comet assays are one of the most common tests for genotoxicity. The technique involves lysing cells using detergents and salts. The DNA released from the lysed cell is electrophoresed in an agarose gel under neutral pH conditions. Cells containing DNA with an increased number of double strand breaks will migrate more quickly to the anode. This technique is advantageous in that it detects low levels of DNA damage, requires only a very small number of cells, is cheaper than many techniques, is easy to execute, and quickly displays results. However, it does not identify the mechanism underlying the genotoxic effect or the exact chemical or chemical component causing the breaks.
Genotoxic effects such as deletions, breaks and/or rearrangements can lead to cancer if the damage does not immediately lead to cell death. Regions sensitive to breakage, called fragile sites, may result from genotoxic agents (such as pesticides). Some chemicals have the ability to induce fragile sites in regions of the chromosome where oncogenes are present, which could lead to carcinogenic effects. In keeping with this finding, occupational exposure to some mixtures of pesticides are positively correlated with increased genotoxic damage in the exposed individuals. DNA damage is not uniform in its severity across populations because Individuals vary in their ability to activate or detoxify genotoxic substances, which leads to variability in the incidence of cancer among individuals. The difference in ability to detoxify certain compounds is due to individuals’ inherited polymorphisms of genes involved in the metabolism of the chemical. Differences may also be attributed to individual variation in efficiency of DNA repair mechanisms. The metabolism of some chemicals results in the production of reactive oxygen species, which is a possible mechanism of genotoxicity. This is seen in the metabolism of arsenic, which produces hydroxyl radicals, which are known to cause genotoxic effects.
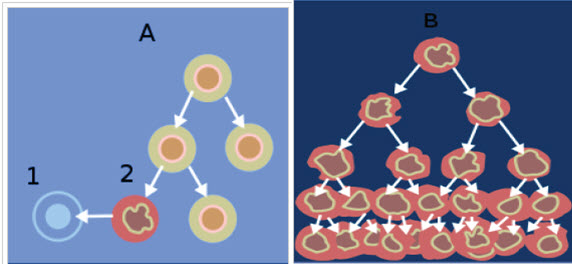
Figure-6: When normal cells are damaged beyond repair, they are eliminated by apoptosis (A).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Cancer cells avoid apoptosis and continue to multiply in an unregulated manner (B)
Genotoxic Chemotherapy
Genotoxic Chemotherapy is the treatment of cancer with the use of one or more genotoxic drugs. The treatment is traditionally part of standardized regime. By utilizing the destructive properties of genotoxins treatments aims to induce DNA damage into cancer cells. Any damage done to a cancer is passed on to descendent cancer cells as proliferation continues. If this damage is severe enough, it will induce cells to undergo apoptosis. A drawback of treatment is that many genotoxic drugs are effective on cancerous cells and normal cells alike. Selectivity of a particular drug's action is based on the sensitivity of the cells themselves. So, while rapidly dividing cancer cells are particularly sensitive to many drug treatments, often normal functioning cells are affected. Another risk of treatment is that, in addition to being genotoxic, many of the drugs are also mutagenic and cytotoxic. So the effects of these drugs are not limited to just DNA damage. In addition, some of these drugs that are meant to treat cancers are also carcinogens themselves, raising the risk of secondary cancers, such as leukemia. [4]
This figure depicts different genotoxic based cancer treatments along with examples.
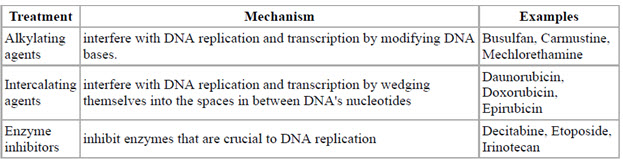
Figure-7: Genotoxic based cancer treatments
Regulatory Genetic Toxicology
• International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
• Regulatory and pharmaceutical representatives from US, Europe & Japan
• Labor intensive process for completion of genetic toxicology guidances (1992 -1997)
• Office of Economic and Cooperation Development (OECD)
• FDA/CDER
• ICH M3 –Guidance for Industry: Non-clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals
* Prior to first human exposure
In vitro tests for the evaluation of mutations and chromosomal damage are generally needed. If an equivocal or positive finding occurs, additional testing should be performed.
* Prior to the initiation of Phase II studies
The standard battery of tests for genotoxicityshould be completed
• FDA/CDER
* S2A Guideline for Industry: Specific Aspects of Regulatory GenotoxicityTests for Pharmaceuticals.
Federal Register 61:18199, 1996
* S2B Guidance for Industry: A Standard Battery for GenotoxicityTesting of Pharmaceuticals.
Federal Register 62:62472, 1997.
Purpose of Genotoxicity Test Assays
• Assays allow detection of a drug’s potential for genotoxicityearly in drug development
• Assays are inexpensive, have high statistical power, are generally reproducible and detect a variety of genotoxicend-points
• Assays designed to be more sensitive to damage in order to enhance hazard identification
Standard Genotoxicity Test Battery
• An in vitrotest for gene mutation in bacteria.
• An in vitrotest with cytogeneticevaluation of chromosomal damage with mammalian cells or an in vitro mouse lymphoma thymidinekinase(tk) assay. [5]
• An in vivotest for chromosomal damage using rodent hematopoieticcells

Figure-8: It displays the standard genotoxiciy test

Figure-9: It displays the thymidine kinase assay
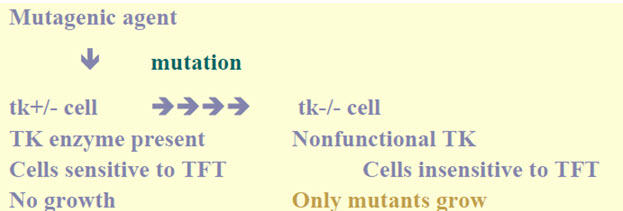
Figure-10: Mouse lymphoma colonies soft agar plates
Figure-11: In-vitro cytogenics assay

Figure-12: CHO-CBL cell karyotypes
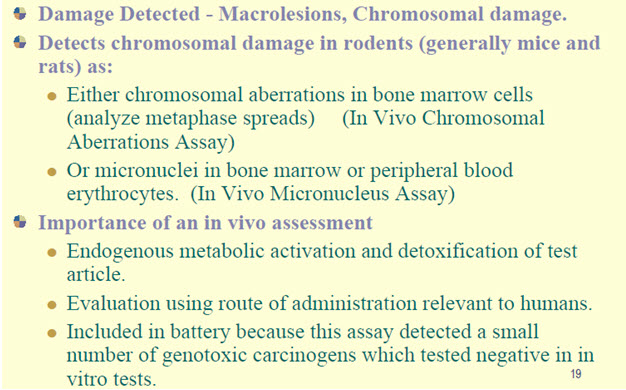
Figure-13: It displays the in-vivo cytogenic assay
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE

Figure-14: In-vivo micronucleus assay
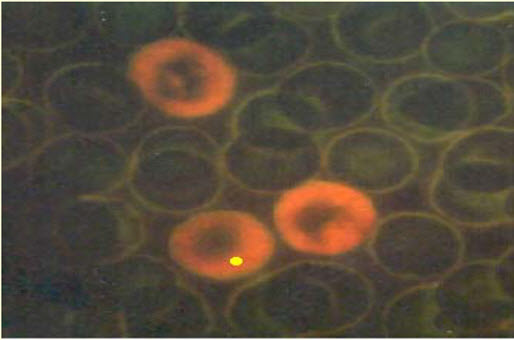
Figure-15: Micronucleated PCE Acridine organ stain
Importance of genotoxicity testing:
Regulatory authorities all over the world require data on the genotoxic potential of new drugs, as part of the safety evaluation process. The pre-clinical studies are generally conducted to obtain the basic toxicological profile of new chemical entities (NCE). The toxicological data are used to evaluate the safety and efficacy of NCE, which will help in predicting the drug's likely risk/benefit assessment in New Drug Application (NDA) process. Genotoxicity assays have become an integral component of regulatory requirement. In addition to it, many people in India are not aware of genotoxicity that it has now become mandatory to include it in drug master file required by European and United States regulatory authorities. Genotoxicity testing of new chemical entities (NCE) is generally used for hazard identification with respect to DNA damage and its fixation. These damages can be manifested in the form of gene mutation, structural chromosomal aberration, recombination and numerical changes. These changes are responsible for heritable effects documented that somatic mutations can also play an important role in malignancy These tests have been used mainly for the prediction of carcinogenicity and genotoxicity because compounds, which are positive in these tests, have the potential to be human carcinogens and/or mutagens. [6-8]
Classification of carcinogens:
The existing systems of classification of carcinogens should include a distinction between genotoxic and non-genotoxic chemicals. For non-genotoxic chemicals, permissible exposure levels can be derived at which no relevant human cancer risks are anticipated. While genotoxic carcinogens can induce chromosomal effects without mutagenic action, non-DNA-reactive genotoxins affecting topoisomerase or the spindle, or those having an exclusively aneugenic effect can be carcinogenic only at high, toxic doses. Specific mechanisms of clastogenicity and processes of carcinogenesis based on reactive oxygen have practical thresholds. Since reactive oxygen species (ROS) are generally genotoxic, the question is whether chemicals that increase ROS production will add to endogenously produced background levels and lead to nonlinear dose-effect relationships. Taking into account the presence of endogenous carcinogens, it is now becoming evident that carcinogenic risk extrapolation to low doses must be considered according to the mode of action.
Genotoxicity, especially when of local nature may only be relevant under conditions of sustained local tissue damage and the associated increased cell proliferation. Cases in point are formaldehyde and vinyl acetate. Defining practical thresholds and health-based exposure limits for these two compounds may prove justified. High doses of reactive oxygen species (ROS) or ROS promoters are clearly toxic. ROS are involved in many forms of tissue damage such as ischemia-reperfusion, atherosclerosis, radiation injury, aging and carcinogenesis. Generally, "oxidative stress" is an important mechanism of indirect genotoxicity that is triggered by exposure to exogenous factors such as UV, ionising radiation, anoxia and hyperoxia. Other pathways are mediated by chemicals producing reactive oxygen species .Paraquat and certain oxidants (potassium bromate, hydrogen peroxide) are the classical examples in this respect. Other exogenous sources of ROS are tobacco smoke, fatty acids, transition metals, ethanol, and redox cycling compounds or physical irradiation by multiple sources. ROS interact with critical molecules within cells and with intracellular signalling, leading to cell death, mutagenesis and toxicities associated with lipid peroxidation. Increased oxidative stress and excessive ROS production cause damage to DNA modifying the base and altering DNA strands, and can contribute to cancer. [9]
Agents capable of causing direct or indirect damage to DNA
• Electrophilic species forming covalent adducts to DNA
- E.g. alkylatingagents
- Arylnitrenium ions
- Diol epoxides of PAH
• UV and ionising radiations
• Reactive oxygen species
• Topoisomerase inhibitors
• Nucleoside analogues
• Protein synthesis inhibitors
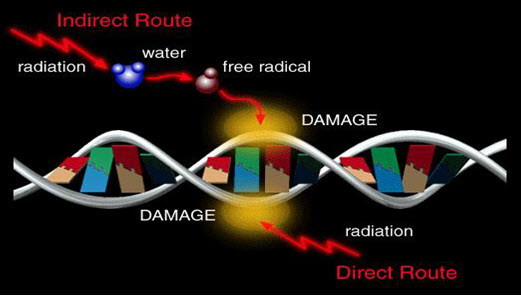
Figure-16: Mechanism of damage to DNA
Classification of carcinogens:
• Carcinogen category 1-shown to cause cancer in humans
• Carcinogen category 2-causes cancer in animal tests, and most probably also in humans
• Carcinogen category 3-possibly carcinogenic, but evidence supporting carcinogenicity is inadequate for the classification to category 2.
IARC (international agency for research on cancer) classification of carcinogens:
• IARC class 1-The substance is carcinogenic to humans.
• IARC class 2A-The substance s probably carcinogenic to humans.
• IARC class 2B-The substance is possibly carcinogenic to humans.
• IARC class 3-The substance is not classifiable to as to its carcinogenicity to humans.
• IARC class 4-The substance is probably not carcinogenic to humans.
Potency of the carcinogen
• TD25 value
• used for example in the setting of EU OELs for genotoxic carcinogens
• TD25/1000 is considered as an acceptable risk level for genotoxic carcinogens, although also socioeconomic and technical constrains have to be taken into account in the setting of OELs Non-genotoxic carcinogens – setting of OELs
• No-observed-adverse-effect level (NOAEL) or Lowest-observed-adverse-effect-level (LOAEL)
• uncertainty factor [10]
Genotoxic carcinogens
– no threshold, no zero risk
– even if exposure levels in the workplace are below OEL, we cannot say that there isn‟t any risk, because according to the current view even small amounts of genotoxic carcinogens may increase our ”mutation burden” and our susceptibility to cancer
– therefore, minimization of exposure as far as possible is essential
Non-genotoxic carcinogens
– Usually considered to possess a threshold
– for example carcinogens which cause cancer via a mechanism involving chronic injury and regeneration => if the OEL is set at the level in which no chronic tissue injury is seen, the cancer risk can be regarded to be negligible . [11-12]
Conclusion
Genotoxicity is a word in genetics defined as a destructive effect on a cell's genetic material (DNA, RNA) affecting its integrity. Genotoxins are mutagens; they can cause mutations. Genotoxins include both radiation and chemical genotoxins. A substance that has the property of genotoxicity is known as a genotoxin. There are three primary effects that genotoxins can have on organisms by affecting their genetic information. Genotoxins can be carcinogens, or cancer-causing agents, mutagens, or mutation-causing agents, or teratogens, birth defect-causing agents. In most cases, genotoxicity leads to mutations in various cells and other bodily systems. Mutations can lead to a host of other problems, from cancer to a wide variety of different diseases. Mutations can come in many different forms; genetic information can be duplicated, deleted, or inserted.
References
1. Kolle, Susanne (20120601). "Genotoxicity and Carcinogenicity". BASF The Chemical Company.
2. "Genotoxicity: Validated Nonanimal Alternatives". AltTox.org. 20110620.
3. Sugden, Kent D. (2001). "Direct Oxidation of Guanine and 7,8Dihydro8oxoguanine in DNA by a High Valent Chromium Complex: A Possible Mechanism for Chromate Genotoxicty". Chemical Research in Toxicology 14: 1315–1322. doi:10.1021/tx010088+. Retrieved 20130404.
4. Chen, Tao (January 2010). "Genotoxicity of Pyrrolizidine". Applied Toxicology.
5. Mei, Nan (2010). "Metabolism, Genotoxicity, and Carcinogenicity of Comfrey". Journal of Toxicology and Environmental Health 13: 509–526. doi:10.1080/10937404.2010.509013.
6. Furman, Grace (20080417). "Genotoxicity Testing for Pharmaceuticals Current and Emerging Practices" (PDF). Paracelsus, Inc.
7. Končar, Helena (2011). "In Vitro Genotoxicity Testing". National Institute of Biology.
8. Tice, R.R. (2000). "Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing" (PDF). Environmental and Molecular Mutagenesis.
9. Bolognesi, Claudia (June 2003). "Genotoxicity of pesticides: A review of human biomonitoring studies". Mutation Research 543: 251–272.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









