 About Authors:
About Authors:
Saritha. A. Surendran*, Prof.O.G. Vinaya, Santhosh. R. Iyer, Anisha. N.A
Calicut University, College Of Pharmaceutical Sciences,
Govt. Medical College, Calicut,
Kerala, India
*sarithaasurendran@gmail.com
ABSTRACT
The Goal of the present investigation was to design and evaluate gels for topical delivery of water insoluble antifungal agent, Clotrimazole with an aim to increase its penetration through skin. Clotrimazole is a broad spectrum imidazole derivative useful in the treatment of superficial fungal infections. Purpose was to improve the solubility, invitro characteristics and dissolution properties of Clotrimazole by the preparation of its solid dispersion with beta cyclodextrin using kneading method by using different drug carrier ratios. Prepared solid dispersion was evaluated for percent practical yield, drug content uniformity, in vitro dissolution rate, DSC and IR studies. Solid dispersions was optimized based on the release characteristics, and were incorporated into gels. Faster dissolution was exhibited by solid dispersion containing l: l ratio of drug: β-cyclodextrin by kneading method. Gels have gained more importance because the gel-bases formulations are better percutaneously absorbed than creams and ointment bases. The gels were formulated by using Carbopol 940, HPMC, and Methyl cellulose and evaluated for pH, drug content, spreadability, extrudability, viscosity determination and diffusion study. Invitro drug release of clotrimazole solid dispersion incorporated gels was showed that Carbopol gels was showed higher drug release as compared to HPMC, methyl cellulose gels. Optimized gel was then undergone skin irritation test, stability studies, and invitro antifungal test. In conclusion, the optimized gel showed good physicochemical properties, better drug release, and reasonable stability
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1393
INTRODUCTION
Topical drug administration is a localized drug delivery system anywhere in the body through ophthalmic, rectal, vaginal and skin as topical routes.Topical drug delivery can be defined as the application of a drug containing formulation to the skin to directly treat cutaneous disorders (e.g. acne) or the cutaneous manifestations of a general disease (e.g. psoriasis) with the intent of containing the pharmacological or other effect of the drug to the surface of the skin or within the skin.Skin is one of the most readily accessible organs on human body for topical administration and is the main route of topical drug delivery system. It affords to maintain applied preparation intact for a prolonged time and this has resulted in its increasing use as a route of administration whether for local, regional or systemic effects.
Topical antifungals are the agents, meant for topical use for fungal infection. Topical application of drug at the affected site offers potential advantage of delivering drug directly to the site of action. Local infection can be treated by application of products which forms transparent water vapours and air permeable film over the skin surfaces, from which drug releases continuously to the skin site and skin structure infection and the disease of the patient would be treated. At present, there are number of antifungal agents used in topical applications like clotrimazole, griseofulvin, itraconazole, fluconazole etc.
Clotrimazole was used as a model drug. Clotrimazole is an imidazole derivative with a broad spectrum antimycotic activity. It acts by inhibiting biosynthesis of ergosterol, an important component of fungal cell membranes. It is widely used for the treatment of localcandidiasis, vaginal yeast infections; topical applications include fungal infections such as ring worm, athlete’s foot and jock itch.Its action leads to increased membrane permeability and apparent disruption of enzyme systems bound to the membrane. The major drawback of this drug is its insolubility in water.
The techniques generally employed to enhance the solubility of poorly water-soluble drugs are, use of surface-active agent, hydrates and solvates, polymorphism, complexation, solid dispersion. Among this Solid dispersion is a unique technique used to increase solubility, dissolution and bioavailability of poorly water-soluble drugs. Conventional method for preparing solid dispersion includes solvent wetting method, physical mixture, complex formations, and solvent evaporation techniques.
Creams, gels, ointments and pastes are some of the topical semisolids in use for many decades. The extensive studies on release properties have revealed that the active ingredients in gel based formulations are better percutaneously absorbed than cream or ointment bases.
[adsense:468x15:2204050025]
MATERIALS AND METHODS
Materials
Clotrimazole I.P. (Gift sample from Chethana Pharmaceuticals, Perinthalmanna, Kerala), β cyclodextrin , Carbopol 940, hydroxyl propyl methyl cellulose, methyl cellulose, Methyl paraben (Hi media Laboratories pvt.Ltd , Mumbai),Propylene glycol (Lobha chemie pvt.ltd, Mumbai),Triethanolamine, Ethanol (Sd fine – chem. Ltd,Mumbai
Methods
Preparation of Clotrimazole solid dispersion
Solid dispersions of clotrimazole in β cyclodextrin containing three ratios (1:1, 1:2, 1:3 w/w) were prepared by kneading method. Here betacyclodextrin was taken in mortar and little amount of ethanol was added and triturated to obtained a homogenous slurry like consistency. Slowly the drug was incorporated into the slurry, and trituration was continued for 1 hour and then dried at 250 c for 24 hours, pulverized, sieved through mesh no.100.The resultant formulations were stored in desiccators until further investigation.
Characterization of Clotrimazole solid dispersion
Percent Practical Yield
Percentage practical yield were calculated to know about percent yield, thus it helps in selection of appropriate ratios of solid dispersion .Solid dispersions were collected and weighed to determine practical yield from the following equation
Percentage Yield = Practical Mass (SD) * 100
------------------------------------
Theoretical Mass (Drug +carrier)
Drug Content
Preparation equivalent to 50 mg of model drug was weighed accurately and dissolved separately in 50 ml methanol. The solutions were further diluted and absorbance of solutions was determined at 261 nm by UV spectrophotometer.
Fourier Transform Infrared spectroscopy
FT-IR spectra of clotrimazole beta-cyclodextrin solid dispersions were obtained by Perkin-Elmer FT-IR spectrophotometer using potassium bromide (KBr) pellets. KBr pellets were prepared by gently mixing the sample with KBr (1:100). The sample was scanned from 4,000 to 400 cm-1.
Differential Scanning Calorimetry
Differential scanning calorimetry was performed by Differential scanning calorimeter shimadzu to obtain suitable thermograms. The accurately weighed sample was placed in an aluminium pan and an empty aluminium pan was used as reference. The experiment was performed under nitrogen flow, at a scanning rate 10 0c/min. in range of 40 - 200 0C
Invitro Dissolution Studies
The USP dissolution apparatus (Type?II) was used for evaluation of in vitro release profile of solid dispersions. The dissolution medium was 900ml phosphate buffer of pH 7.4 kept at 37 ± 0.1°. Solid dispersion was taken in muslin cloth and then kept in the basket of dissolution apparatus, which was then rotated at 100 rpm. Samples of 5ml were withdrawn at specified time intervals and analyzed spectrophotometrically at 262 nm. Withdrawn samples were replaced by fresh buffer solution. Each preparation was tested in triplicate and then means values were calculated.
Preparation of Clotrimazole solid dispersion incorporated gels
The polymer and purified water I.P. were taken in a mortar and allow soaking for 24 hrs. Solid dispersion containing required amount of drug was dissolved in 95% ethanol and other additives were added and the trituration was continued until the homogenous gel was formed
|
Ingredi |
Carbopol gel |
HPMC gel |
Methyl cellulose gel |
||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
|
|
Clotrimazole I.P containing solid dispersion |
1% |
1% |
1% |
1% |
1% |
1% |
1% |
1% |
1% |
|
Carbopol 940 |
0.4gm |
0.5gm |
0.6gm |
- |
- |
- |
- |
- |
- |
|
HPMC |
- |
- |
- |
2gm |
4gm |
7gm |
- |
- |
- |
|
Methyl cellulose |
- |
- |
- |
- |
- |
- |
1gm |
3gm |
6gm |
|
Propylene glycol |
10ml |
10ml |
10ml |
10ml |
10ml |
10ml |
10ml |
10ml |
10ml |
|
Triethanol amine |
0.5ml |
0.5ml |
0.5ml |
0.5ml |
0.5ml |
0.5ml |
0.5ml |
0.5ml |
0.5ml |
|
Methyl paraben |
0.03gm |
0.03gm |
0.03gm |
0.03gm |
0.03gm |
0.03gm |
0.03gm |
0.03gm |
0.03gm |
|
Ethanol |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
2.5ml |
|
Purified water upto |
100gm |
100gm |
100gm |
100gm |
100gm |
100gm |
100gm |
100gm |
100gm |
Evaluation and optimization of Clotrimazole solid dispersion incorporated topical gels
Physical appearance and homogeneity
Gel formulation containing clotrimazole were visually inspected for clarity, colour, homogeneity, presence of particles and fibers.
Determination of pH
The pH of gels was checked by using a digital Elico pH meter at room temperature. Initially, the pH meter was calibrated using standard buffers of pH 4 and 9.2. Accurately 2.5gm of gel was weighed and dispersed in 25 ml of purified water and then pH meter was dipped in the dispersion and the pH was noted
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Determination of viscosity
The viscosity of gels was determined by using Brookfield (DV—II+) viscometer.
Determination of spreadability
Two glass slides of 20 * 20 cm each were selected. The formulation whose spreadability had to be determined (500 mg) was placed over one of the slides. The second slide was placed over this slide in such a way that the formulation was sandwiched between the two slides. 100 gm weight was placed upon the upper slide so that the formulation between the two slides was pressed uniformly to form a thin layer. The weight was removed and the excess of the formulation adhering to the slides was scrapped off. The lower slide was fixed on the board of the apparatus and the upper slide was tied to a string to which 20 gm load could be applied with the help of a simple pulley. The time taken for the upper slide to travel the distance of 6 cm and separate away from lower slide under the direction of the weight was noted. The experiment was repeated and the average of three such determinations was calculated for each formulation.
Spreadability was then calculated using the following formula
= M × L/ T
Where, S = is the spreadability, M = is the weight in the pan (tied to the upper slide), L = is the length moved by the glass slide and T = represents the time taken to separate the slide completely from each other.
Determination of extrudability
For a good gel formulation, it should extrude easily from the container. In this test, sample is extruded from the tube by usual procedure. A closed collapsible tube containing gel was passed firmly at crimped end. When the cap was removed, gel extrudes until pressure was dissipates. The weight in grams required to extrude 0.5 cm ribbon of gel in 10 seconds was determined. The results for each formulation were recorded as extrusion pressure in grams.The gram of the gel extruded was calculated and grades were allotted (++ good; + fair)
Drug content
1 gm gel equivalent to 10 mg were taken and dissolved separately in 10ml methanol. The solutions were further diluted and absorbance of solutions was determined at 262 nm by UV spectrophotometer. The actual drug content was calculated.
Invitro drug diffusion studies
In vitro diffusion study through fresh human skin was performed using a Franz type diffusion cell at 32 ± 0.5 0c .Fresh human skin was obtained from the plastic surgery department in Medical College, Calicut. Fresh human skin was mounted between the donor and receptor compartments. I gm gel was placed on the skin and the compartments clamped together. The diffusion cell was filled with 25 ml of phosphate buffer solution (pH 7.4). The whole assembly was fixed on a magnetic stirrer and the solution was continuously stirred at 50 rpm. The samples are withdrawn at 1, 2,3,4,6 and 8 hrs and analyzed for drug content by UV spectroscopy at 262nm. The receptor phase was replenished with an equal volume of phosphate buffer solution (pH 7.4): methanol at each sample withdrawal.
Evaluation of optimized clotrimazole solid dispersion incorporated gels Invitro antifungal studies
The anti-fungal activity of the optimized Clotrimazole solid dispersion incorporated gel was compared with marketed clotrimazole gel, was determined using Candida albicans. The suspension of Candida albicans was poured evenly on plates of Sabourauds dextrose agar. Agar wells were cut from the seeded agar medium using a sterile cork borer. These wells were filled with 10 mg of formulated gel prepared with 1% drug and also without drug. Moreover, 10 mg of marketed clotrimazole gel (1%) was used for comparison. Plates were then incubated at 37?C for about 3 days and compared the zone of inhibitions. The bases without clotrimazole (blank gel), and pure clotrimazole solution were used as controls.
Skin irritation test
The skin irritation test was performed on healthy white rabbit of average weight 1.75 to 2.25 kg. About 9 cm2 area on the dorsal surface of the rabbits in each group was shaved and cleaned with spirit. Rabbits were divided into three groups (n = 3) as follows:
Group - I (control): - There was no application on the surface of the rabbit skin.
Group –II (marketed gel)):- 1 gm of the gel containing 10 mg of clotrimazole was applied to 9cm2 area on the dorsal surface of the rabbit. The visual inspection was observed for 3 days to check evidence of skin irritation (sign of edema and erythema).
Group - III (Formulated gel, F2):- 1 gm of gel containing 10 mg of clotrimazole was applied to 9cm2 area on the dorsal surface of the rabbit. The visual inspection was observed for 3 days to check any evidence of skin irritation (sign of edema and erythema) Skin irritation test was conducted to evaluate the irritancy of the optimized clotrimazole gel on the intact skin of rabbits.
RESULTS AND DISCUSSION
Clotrimazole Solid Dispersion
All the Solid dispersions prepared were found to be fine and white colored free flowing powders.
Percent practical yield
The results of percent practical yield studies are shown in Table1. The % Practical yield of the prepared solid dispersions was found to be in the range of 88.2 ?92.0%. The maximum yield was found 92.0% in S1 formulation.
Drug content
The actual drug content of all the 3 formulations is shown in Table 1. The drug content of the prepared Solid dispersions were in the range of 83.4 ?95.0% indicating the application of the present methods for the preparation of Solid dispersion with high content uniformity. The maximum % drug content was found 95.0% in S1 formulation
Table.1 Characteristics of Clotrimazole solid dispersion
|
Solid dispersion code |
Drug content % |
% Practical yield |
|
S1 |
95.0 |
92.0 |
|
S2 |
87.0 |
90.3 |
|
S3 |
83.4 |
88.2 |
Invitro dissolution study
The in vitro release studies of different batches of solid dispersions are shown in figure 1. The solid dispersion prepared by kneading method showed improved dissolution. Among the solid dispersions prepared 1:1 ratio showed greater solubility than the others. Because of enhanced/ greater release solid dispersion prepared with 1:1(S1) drug carrier ratio was selected as ideal batch for incorporation into gels.
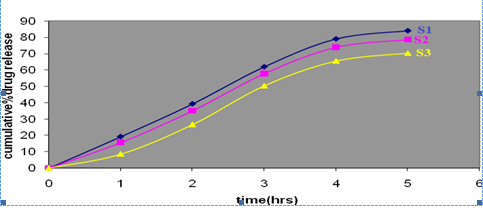
Fig.1 Percent release of Clotrimazole solid dispersions
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
IR spectroscopy
IR studies indicated that no chemical interaction between drug and carrier took place during preparation of solid dispersion of clotrimazole
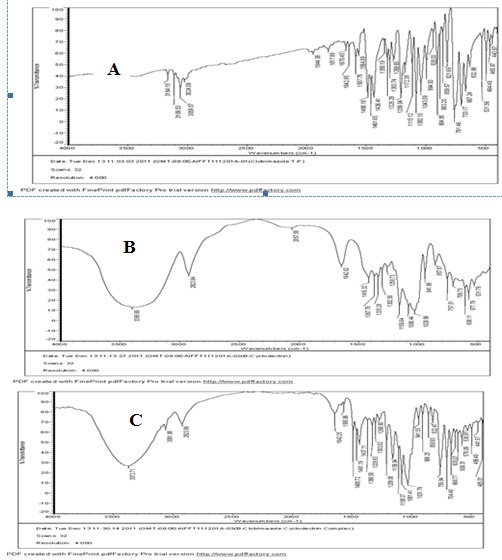
Fig.2 IR spectra of Clotrimzole (A), β cyclodextrin (B), clotrimazole-β cyclodextrin complex – S1 (C)
Differential Scanning calorimetry
When guest molecules are incorporated in the ß?cyclodextrin cavity or in the crystal lattice, their melting, boiling, and sublimation points usually are shifted to a different temperature or disappear within the temperature range in which the ß?cyclodextrin lattice is decomposed. In clotrimazole β cyclodextrin inclusion complex DSC plot, two melting ranges were obtained. In one peak, melting process started at 80.37 0C and completed at 111 0C. In another peak, process was started at l41.96 0C and completed at 148.42 0C was suggested that clotrimazole and β cyclodextrin used for the formulation was nothing but a mixture of both.
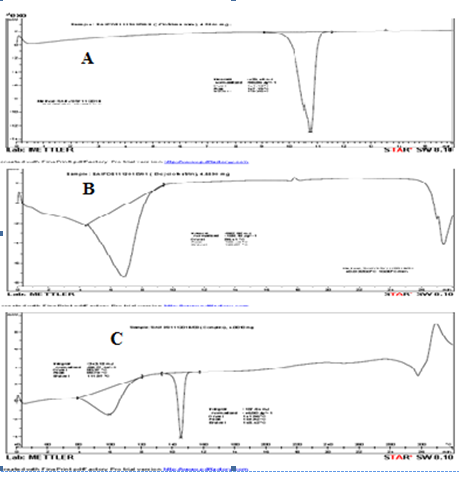
Fig.3 DSC thermogram Clotrimazole (A), β cyclodextrin(B), Clotrimazole β cyclodextrin complex- S1 (C)
Clotrimazole solid dispersion incorporated gels
Physical characteristics of Clotrimazole solid dispersion incorporated gels were measured according to the methods describe above. The results are listed in table 2
Table 2. Physical Characteristics of Clotrimazole solid dispersion incorporated gels
|
Formulation code |
Physical appearance |
pH |
Homog |
Viscosity (cps) |
Spreadability |
Extrud |
% Drug content |
|
F2 |
White tanslucent |
6.83 |
+++ |
7630 |
13.60 |
++ |
95 |
|
F5 |
White tanslucent |
6.75 |
++ |
5450 |
14.75 |
++ |
95 |
|
F8 |
White tanslucent |
6.79 |
++ |
2450 |
15.25 |
++ |
95 |
++ + Excellent ++ good
Invitro diffusion studies
The in vitro diffusion studies were performed by over a period of 8 hours and results are shown in figure 4.F2 was showed better release (73.88 ± 0.42)
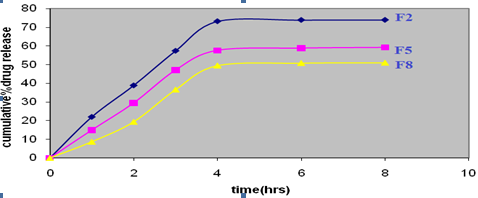
Fig. 4 Diffusion profile of Clotrimazole Solid dispersion incorporated gels
Optimized Clotrimazole solid dispersion incorporated gels Invitro antifungal study
Invitro antifungal activity of optimized gel F2, marketed clorimazole gel M, blank gel B , control C were determined by cup plate method using Candida albicans as test organisms and Zone of inhibition was measured and compared.
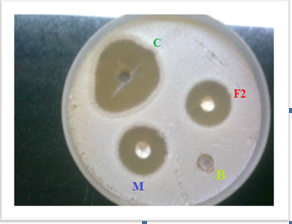
Fig: 5 Compared antifungal activity of optimized Clotrimazole gel F2 with control and marketed preparation
Skin irritation test
The optimized formulation F2 was subjected for skin irritation test. The Skin irritation tests were performed on healthy white rabbits and were observed for a period of 72 hrs. From the results of the study it was clear that there is no erythema and edema found after 72 hrs, and hence it was concluded that the gel was free from skin irritation.
CONCLUSION
Invitro drug release of clotrimazole solid dispersion incorporated gels was showed, (F2) found the best release compared to other formulated gels and also undergone skin irritation test, and invitro antifungal test.
It can be concluded that the optimized gel showed good physicochemical properties, better drug release, uniform texture and reasonable stability. Since the results are encouraging for the optimized formulation, F2, the proper technique can be applied for commercial and mass production.
REFERENCES
1. Dipen.M.Patel, Kavitha.K; Formulation and Evaluation Aspects of Transdermal delivery system; International Journal of Pharmaceutical sciences Review and Research; vol.6; ( 2); January – February 2011; article-01; 83 – 90
2. Balakrishnan Prabagar, Bong-Kyu yoo, Jong-Ae kim,et al; Enhanced bioavailability of poorly water soluble clotrimazole by inclusion with betacyclodextrin; Arch Pharm; 2007; feb.30; (2); 249-254
3. Priya Batheja, Larsia Sheihet, et al; topical drug delivery by a polymeric nanosphere gel: Formulation ,optimization and invivo and invitro skin distribution studies; Journal of controlled release; (2011); 149;159 – 167
4. Rachit Khullar,et al; Formulation and evaluation of mefenamic acid and emulgel for topical delivery; Saudi pharmaceutical journal; (2012); 20;63-67
5. Dangi Amish.A, etal ;Formulation and evaluation once daily mucoadhesive vaginal tablet of clotrimazole using natural and synthetic polymers; Asian journal of pharmaceutical and health sciences; oct – dec (2011); 176-182
6. B.Niyaz Basha, et al; Formulation and evaluation of gel containing Fluconazole antifungal agent;International journal of drug development &research ; oct –dec (2011); 3; 4; 109 -128
7. Praveen.S.Patil et al ; Development and evaluation of anti – dandruff hair gel; International journal of research in pharmacy and chemistry; 2011;1; (4); 936-944
8. Mohammed Jafar, et al; Studies on meloxicam solid dispersion incorporated buccal patches; international research journal of pharmacy; 2011; 1;(5); 220 -227
9. P.K.Lakshmi ,et al; Formulation and evaluation of ibuprofen topical gel; A novel approach for penetration enhancement; International journal of applied pharmaceutics; 2011; 3; (3); 25 -30
10. B.V.Miitkari et al; Formulation and evaluation of topical liposomal gel for fluconazole; Indian journal of pharmaceutical education and research ;oct – dec 2010; 44; (4)
11. K.Purushotham Ra et al; Design of clotrimazole soap bars for skin diseases; International journal of current pharmaceutical research; 2011; 3; (4); 41- 45
12. Aejaza et al; studies of acelophenac solid dispersion incorporated gels : development, characterization and in vitro evaluation; International journal of pharmacy and pharmaceutical sciences; 2010;1; 111 – 115
13. Mostafa shahin et al; Optimized formulation for topical administration of clotrimazole using Pemulen polymeric emulsifier; Drug development and Industrial pharmacy; 2011; 37; (5); 559-568
14. M.Najmuddin, et al; Formulation and evaluation of solid dispersion incorporated gel of ketoconazole; Research Journal of Pharmaceutical, Biological and Chemical Sciences; April –June 2010; vol.1; (2); 406 -412
15. Boushra.M.El-Houssieny et al ;Formulation and evaluation of clotrimazole from pluronic F127 gels; drug discoveries and therapeutics;2010;4;(1); 33-43
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









