About Authors:
S. H. Seyed Mohamed Buhary*, Kamarapu Nagaraju, A. Thanga Thirupathi.
Department of Pharmaceutics, Sankaralingam Bhuvaneswari College of Pharmacy,
Sivakasi, Tamil Nadu, India.
* nagarajukamarapu8@gmail.com
ABSTRACT:
The main goal of this study was to develop a stable formulation of antibiotic drug clarithromycin as an immediate-release tablet. The task of developing immediate release tablet is accomplished by using a suitable diluents and super-disintegrants. Faster disintegration of the tablet administrated orally minimizes absorption time and improves its bioavailability in less time. The formulation development work was initiated with wet granulation. Microcrystalline cellulose PH 102 were used as diluent. Povidone was used as the binder. Croscarmellose sodium and pre gelatinized starchwas added as a disintegrating agent. Talc and magnesium stearate was used as the lubricant. The prepared granules were compressed into a compression machine. To evaluate the formulated tablets as per requirements of standards. The tablets thus formulated showed a satisfactory physical parameters, and it was found to be stable.Evalution Parameters Like weight variation, hardness of the tablet, friability, thickness, disintegration test, drug content uniformity and in vitro release studies were performed.To optimize the trial batch by 32 full factorial design study and determined the best batch by in vitro release studies are showed that optimization formulation (OF7) was 101.62% respectively. The optimized formulation is further selected and compared with the release profile of the innovator product. The results suggest the feasibility of developing immediate tablets consisting of clarithromycin for the convenience of patients with respiratory infections, gonorrhea, community-acquired pneumonia, pelvic inflammatory disease, pediatric otitis media and pharyngitis and Mycobacterium avium complex (MAC) in patients with advanced HIV disease.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1395
INTRODUCTION:
Immediate release drug delivery system are based on single or multiple-unit reservoir or matrix system, which are designed to provide immediate drug levels in short period of time. Immediate release drug delivery is desirable for drugs having long biological half life, high bioavailability, lower clearance and lower elimination half life. Oral drug delivery is the most desirable and preferred method of administering therapeutics agent for their systemic effect. In addition, the oral medication is generally considered as the first avenue investigated in the discovery and development of new drug entities and pharmaceutical formulation, mainly because of patient acceptance, convenience in administration and cost effective manufacturing process.
Clarithromycin is a macrolide antibiotic with broad spectrum of activity. It is given in the treatment of respiratory tract infections, in the skin and soft tissue infections. Clarithromycin may be given to eradicate H. pylori in treatment regimens for peptic ulcer diseases. Clarithromycin has in vitro antimicrobial activity against typical (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis) and atypical (Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila) pathogens commonly associated with community-acquired lower respiratory tract infections.
Clarithromycin is rapidly absorbed from the GIT and undergoes first pass metabolism. The bioavailability of the drug is about 55%. The terminal half-life of Clarithromycin is reportedly about 3-4 hours. Compared with erythromycin, clarithromycin possesses greater acid stability, improved pharmacokinetic properties, and fewer gastrointestinal side effects. It has a narrow absorption window in gastrointestinal tract, rapid gastrointestinal absorption, highly soluble at acidic pH,absorption of clarithromycin is unaffected by food. More than half of an oral dose is systemically available as the parent drug and the active 14-hydroxy metabolite. Pharmacokinetics are nonlinear, with plasma concentrations increasing in more than proportion to the dosage. First-pass metabolism results in the rapid appearance of the active metabolite 14-hydroxy-clarithromycin in plasma. Clarithromycin and its active metabolite are found in greater concentrations in the tissues and fluids of the respirator, it has higher eradication rate in vivo to H. pylori. The recommended dosage regimen for these types of infections in adult patients is 250 to 500 mg twice daily for 7-14 days of the immediate-release oral formulation of clarithromycin.1,2,3
[adsense:468x15:2204050025]
MATERIALS AND METHODS
Materials
Clarithromycin was procured as a gift sample from Anuh pharma private limited, Mumbai, India. Croscarmellose sodium procured as a gift sample from signet chemical company, Mumbai, India.Pregelatinised starchwas procured from DMV Fonterra excipients, USA.Avicel PH102, brand name of MCC supplied byWeiming Pharmaceuticals, Taipei, Taiwan.Klucel, brand name ofHydroxy propyl cellulose supplied byAqualon, Wilmington, USA.All other ingredients were of laboratory grade.
METHODS
Preparation of Clarithromycin Immediate Release Tablets
Clarithromycin 500 mg was mixed with required quantities of 2.0% croscarmellose sodium was for 3-5 minutes, the blend was granulated mechanically by Kneading method, using 4.11% Povidone in Isopropyl alcohol as binder, the wet coherent mass was dried in hot air oven at 60oC until the moisture content of granules is NMT 1% and passed through sieve # 20, the granules were mixed with Croscarmellose sodium, Pregelatinized starch and microcrystalline cellulose PH 102 are mixed for 4-5 minutes.
The above powder blend with specified quantity of 1.11% Talc, 1.11% Aerosil are mixed homogenously for 2-3 minutes and finally 0.8% Magnesium stearate are mixedwas done manually using polyethylene bag for a 1-2 minutes.The physical properties (Bulk density, Tapped density, Compressibility index, and Hausner’s ratio) of the mixtures were evaluated and shown in Table 5. The granules were compressed on 8 station rotary tablet press (Accura, Ahmedabad, India) using 19.5 X 9.5 caplet punches. The weights of the tablets were kept constant for all formulations, which were 850 mg for formulation F1 to formulation F8.
The tablets were ovoloid shaped with an average length of 19.4±0.1 mm and average thickness of 6.0±0.1 mm with a minimum of 500 tablets were prepared for each batch. These uncoated tablets are selected for 3.0% film coating to the weight of uncoated tablets using Hydroxy propyl methyl cellulose 15 cps, Ethyl cellulose, titanium dioxide, Talc, Quinoline yellow lake, Ethyl vanillin, Propylene glycol, Dichloromethane and Isopropyl alcohol. The formulations of the all batches are shown in Tables 2. Drug-polymer interactions were determined by IR spectra and shown in figures 1.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
OPTIMIZATION OF TRIAL BATCH (F8) BYFULL FACTORIAL DESIGN 8,9
In order to obtain “best” or an “optimized product” nine different formulations were generated using a 32 randomized full factorial. Based on preformulation study the amounts of croscarmellose sodium (X1) and microcrystalline cellulose PH102 (X2) were selected as the independent factors, studied at 3 levels each (-1, 0, +1). The percentage drug release (y1) and disintegration time (y2) were taken as dependent factors. Experimental trials were performed at all 9 possible combinations of X1 and X2. Batches for factorial design are shown inTable No.1
Table No. 1 : Formulation trials as per experimental design
|
Trial No. |
Coded factor levels |
|
|
X1 |
X2 |
|
|
I |
-1 |
-1 |
|
II |
-1 |
0 |
|
III |
-1 |
1 |
|
IV |
0 |
-1 |
|
V |
0 |
0 |
|
VI |
0 |
1 |
|
VII |
1 |
-1 |
|
VIII |
1 |
0 |
|
XI |
1 |
1 |
|
Translation Of Coded Levels In Actual Units |
|||
|
Coded level |
-1 |
0 |
1 |
|
X1: CCS (%) |
2 |
3 |
4 |
|
X2:MCC102 (%) |
21 |
23 |
25 |
Table No.2: Formulation Trial Batches
|
S.No. |
Ingredients |
F-1 (mg/ |
F-2 |
F-3 |
F-4 |
F-5 |
F-6 |
F-7 |
F-8 (mg/ |
|
1 |
Clarithromycin |
500.00 |
500.00 |
500.00 |
500.00 |
500.00 |
500.00 |
500.00 |
500.00 |
|
2 |
Croscarmellose sodium |
17.00 |
17.00 |
17.00 |
17.00 |
17.00 |
17.00 |
17.00 |
17.00 |
|
4 |
Povidone |
- |
- |
21.00 |
34.25 |
35.00 |
35.00 |
35.00 |
35.00 |
|
5 |
Colloidal silicon dioxide |
- |
- |
- |
- |
6.00 |
8.50 |
8.50 |
- |
|
6 |
Hydroxy propyl |
15.00 |
20.00 |
- |
- |
- |
- |
- |
- |
|
7 |
Microcrystalline cellulose PH101 |
235.70 |
229.30 |
230.00 |
220.00 |
- |
- |
- |
- |
|
8 |
Croscarmellose sodium |
25.50 |
25.50 |
- |
25.50 |
25.50 |
25.50 |
25.50 |
25.50 |
|
9 |
Microcrystalline cellulose PH112 |
- |
- |
39.45 |
- |
- |
- |
- |
- |
|
10 |
Microcrystalline cellulose |
50.00 |
48.00 |
- |
36.00 |
251.50 |
249.00 |
199.00 |
196.00 |
|
11 |
Pregelatinised starch |
- |
- |
- |
- |
- |
- |
50.00 |
50.00 |
|
12 |
Talc |
- |
- |
8.50 |
4.25 |
10.0 |
8.65 |
8.65 |
9.50 |
|
13 |
Colloidal silicon dioxide |
2.55 |
3.82 |
4.30 |
4.50 |
- |
- |
- |
9.50 |
|
14 |
Magnesium stearate |
4.25 |
6.38 |
4.75 |
8.5 |
5.00 |
6.35 |
6.35 |
7.50 |
|
15 |
Isopropyl Alcohol |
- |
- |
- |
q.s |
q.s |
q.s |
q.s |
q.s |
|
16 |
Purified water |
q.s |
q.s |
q.s |
- |
- |
- |
- |
- |
|
Average Weight |
850 |
850 |
850 |
850 |
850 |
850 |
850 |
850 |
|
Table No. 4 : Formulation Trial Batches For Optimized Batches
|
Ingredients |
Formulation Code Qty/Tab (mg) |
||||||||
|
OF1 |
OF2 |
OF3 |
OF4 |
OF5 |
OF6 |
OF7 |
OF8 |
OF9 |
|
|
Clarithromycin |
500 |
500 |
500 |
500 |
500 |
500 |
500 |
500 |
500 |
|
Croscarmellose sodium |
17.0 |
17.0 |
17.0 |
17.0 |
17.0 |
17.0 |
17.0 |
17.0 |
17.0 |
|
Povidone |
35.00 |
35.00 |
35.00 |
35.00 |
35.00 |
35.00 |
35.00 |
35.00 |
35.00 |
|
Croscarmellose sodium |
25.25 |
25.25 |
25.25 |
25.50 |
25.50 |
25.50 |
25.75 |
25.75 |
25.75 |
|
Microcrystalline cellulose PH102 |
194.5 |
196.5 |
198.5 |
194.5 |
196.5 |
198.5 |
194.5 |
196.5 |
198.5 |
|
Pregelatinised starch |
51.75 |
49.75 |
47.75 |
51.5 |
49.5 |
47.50 |
51.25 |
49.25 |
47.25 |
|
Talc |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
|
Colloidal silicon Dioxide |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
9.50 |
|
Magnesium Stearate |
7.50 |
7.50 |
7.50 |
7.50 |
7.50 |
7.50 |
7.50 |
7.50 |
7.50 |
|
Isopropyl Alcohol |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
|
TOTAL |
850 |
850 |
850 |
850 |
850 |
850 |
850 |
850 |
850 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
EVALUATION OF CLARITHROMYCIN TABLETS
The prepared tablets were evaluated for quality control tests like weight variation, hardness, thickness, friability and content uniformity, in-vitro dissolution studies.
Weight Variation4,5
Twenty tablets were selected at random and average weight was calculated. Then individual tablets were compared with the average weight was compared with an average weight. The results are shown in table 6.
Hardness 4,5
Hardness of tablet was determined by Monsanto hardness tester. The results are shown in table 6.
Friability 4,5
Friability test was done by Roche friabilator. Ten tablets were weighed and were subjected to the combined effect of attrition and shock by utilizing a plastic chamber that revolve at 25 rpm dropping the tablets at distance of 6 in. with each revolution. Operated for 100 revolutions, the tablets were de dusted and reweighed. The percentage friability was calculated. The results are shown in table 6.
Thickness 4,5
The dimensions of the tablet like thickness, length were measured using vernier-calipers. Ten tablets were selected randomly for this test and the average value was reported. The results are shown in table 6.
In-Vitro Dissolution Studies By HPLC6,7
Dissolution system:
Apparatus: Dissolution Apparatus USP Type – II (Paddle), Speed: 50 RPM, Medium: 900 ml of Acetate buffer pH 5.0, Temperature: 37°C ± 0.5°C, Time: 30 Minutes
Procedure :
Preparation of Buffer solution:
13.61 gm of Sodium acetate trihydrate was dissolved in 1L of purified water in 1L volumetric flask and mix. Then Adjust with 0.1M acetic acid to apHof 5.0
Preparation of Standard solution:
62.50 mg of clarithromycin USP working Standard was accurately weighed and transferred in a 100ml volumetric flask and dissolved using methanol, and made up to the volume using dissolution medium.
From the above solution, 10 ml was taken in a 50 ml volumetric flask and made up to a volume with mobile phase.
Preparation of sample solution:
Apparatus was set as per above conditions, one tablet placed in each of the six dissolution vessels and started the dissolution test. After completion of 30 minutes, 20 ml of the solution was withdrawn from dissolution bowl. The filtrate was collected after discarding first few ml of the filtrate. From this 5 ml of the filtrate was diluted to 25 ml with mobile phase.
Chromatographic system:
Apparatus: HPLC, Column: A stainless steel column (250×4.6mm) packed with Octa decyl silane (C18) bonded to porous silica, Flow rate: 1.0 ml/min, Column oven temperature: 50oC, Injection volume: 50µL, Wave length: 210 nm
Preparation of buffer solution:
9.113 gm of monobasic potassium phosphate was accurately weighed and dissolved in 1000 ml of water and its pHwas adjusted to 4.0 with dilute ortho phosphoric acid.
Preparation of mobile phase:
Take 650 ml from the above buffer solution and 350 ml of methanol was mixed well, and filtered and degassed.
Procedure:
50 microlitres of filtered portion of the standard solution and sample solution were separately injected into the HPLC system. The chromatogram was recorded and the responses were measured for the major peaks. The amount of drug release of clarithromycin USP was calculated in percentage with respect to label claim by using the following expression.
% drug released = (AT/AS)×(WS/100)×(10/50)×(900/1)×(25/5)×(P/100)
Where, AT are area of the clarithromycinin sample solution, AS are average area of the clarithromycinpeak for standard solution, WS are weight taken in mg for standard solution, P are percent purity of clarithromycinon as such basis, LA are labeled amount of clarithromycin respectively.
System suitability:
1. % RSD of five replicate injections peak should not be more than 2.0%
2. The theoretical plate for clarithromycin peaks should not be less than 750.
3. The tailing factor for clarithromycin peaks should not be more than 2.0
Assay (By HPLC)6,7
Preparation of buffer solution:
9.113 gm of monobasic potassium phosphate was accurately weighed and dissolved in 1000 ml of water and its pH was adjusted to 4.0 with dilute ortho phosphoric acid.
Preparation of mobile phase:
650 ml of the above buffer solution and 350 ml of methanol was mixed well, filtered and degassed.
Preparation of standard stock solution:
62.50 mg of clarithromycin USP working Standard was accurately weighed and transferred in a 100ml volumetric flask and dissolved using methanol, and made up to the volume using mobile phase.
Preparation of standard solution:
10 ml of standard stock solution was taken in a 50ml volumetric flask and made up to a volume with mobile phase. This solution contains about 125µg of clarithromycin per ml.
Preparation of resolution solution:
62.5 mg of clarithromycin USP related compound A was weighed in a 50ml volumetric flask and dissolved using methanol. Then 10 ml of this solution and 10 ml of standard stock solution was transferred to a 50 ml volumetric flask and made up to the volume with mobile phase.
Preparation of sample solution:
20 tablets were weighed and crushed to a fine powder, then weighed 438.0 mg of tablet powder (i.e., equivalent to about 250 mg of clarithromycin) was transferred to 200 ml volumetric flask, then about 75 ml of methanol was added and sonicated for 30 minutes. Diluted with methanol to volume and mixed well,and allowed the solution to settle insoluble matter. Then transferred 5ml of supernatant solution to a 50 ml volumetric flask and made up to volume with mobile phase and mixed well. Passed the solution through filter paper and used this solution as assay preparation.
Chromatographic conditions : Column: A stainless steel column (250×4.6mm) packed with Octa decyl silane (C18) bonded to porous silica, Flow rate : 1.0 ml/min, Column oven temperature: 50oC, Injection volume: 50µL, Wave length : 210 nm
Procedure:
50 microlitres of filtered portion of the resolution solution, standard solution and sample solution were separately injected into the HPLC system. The chromatogram was recorded and the responses were measured for the major peaks. The content of per tablet was calculated using the following expression Content of tablet=(AT/AS) ×(WS/100)×(10/50)×(200/WT)×(5/50)×(P/100)×Avg.wt ×100
Where, AS are average area of the clarithromycin peak in standard solution, AT are Area of the clarithromycin peak in sample solution, WS are Weight of clarithromycin taken for standard in g, WT are Weight of clarithromycin taken for sample in g,P are Percent purity of clarithromycin on as such basis respectively.
System suitability:
1. % RSD of five replicate injections peak should not be more than 2.0%
2. The theoretical plate for clarithromycin peaks should not be less than 750.
3. The tailing factor for clarithromycin peaks should NLT 0.9 and NMT 1.5.
4. The resolution between clarithromycin and clarithromycin related compound A should not be less than 2.
Similarity FactorCalculations 10,11,12
For dissolution profile comparison, a model independent mathematical approach to compare the dissolution profile using two factors, f1 and f2.
The difference factor (f1) calculates the percentage difference between the two curves at each time point and is a measurement of the relative error between the two curves f1= {[Σt=1n | Rt – Tt |] / [Σt=1n Rt]} .100
where, n is the number of time points, Rt is the dissolution value of the reference batch at time t and Tt is the dissolution value of the test batch at time t.
The FDA and EMEA defined similarity factor as a "logarithmic reciprocal square root transformation of one plus the mean squared (the average sum of squares) differences of drug percent dissolved between the test and the reference products"
In other words, the similarity factor (f2) is a logarithmic transformation of the sum-squared error of differences between the test Tt and reference products Rt over all time points. It represents closeness of two comparative formulations. Generally similarity factor in the range of 50-100 is acceptable according to US FDA.
f2 = 50. log {[1 + (1/n) Σt=1n (Rt-Tt)2 ] -0.5 .100}
The comparative dissolution study was performed to determine the similarity of dissolution profiles for the immediate release clarithromycin (OF7) between the innovator product.The results are tabulated inTable No. 11.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS
FT-IR spectram of clarithromycin and its excipients.
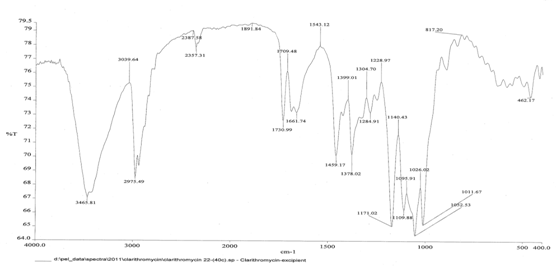
Figure No. 1: FT-IR spectra of clarithromycin and its excipients.
Table No.5: Pre compression parameters for clarithromycin trial batch
|
S. |
Formu |
Bulk density |
Tapped density (gm/cc) |
Carr’s index (%) |
Haus |
Angle |
Moisture content (%) |
|
1 |
F1 |
0.472 |
0.556 |
15.10 |
1.17 |
33.30 |
1.26 |
|
2 |
F2 |
0.484 |
0.574 |
15.67 |
1.18 |
34.93 |
1.05 |
|
3 |
F3 |
0.510 |
0.575 |
11.30 |
1.12 |
33.73 |
0.93 |
|
4 |
F4 |
0.532 |
0.611 |
12.92 |
1.14 |
33.40 |
0.85 |
|
5 |
F5 |
0.486 |
0.563 |
13.67 |
1.15 |
31.43 |
0.83 |
|
6 |
F6 |
0.534 |
0.593 |
9.94 |
1.11 |
31.7 |
0.78 |
|
7 |
F7 |
0.522 |
0.589 |
11.37 |
1.12 |
30.14 |
0.86 |
|
8 |
F8 |
0.532 |
0.595 |
10.58 ±0.02 |
1.11 |
30.91 |
0.84 |
EVALUATION FOR POST COMPRESSION PARAMETERS
Table No. 6: Post compression parameters for clarithromycin trial (F1-F8) batch for uncoated tablets.
|
Formu |
Average |
Thickness (mm)* |
Hardness (kg |
Disinte |
Friab |
Weight variation (mg) |
Assay (%) |
|
F1 |
851 |
6.00 |
9.3 |
1.50 |
- |
850.0 |
- |
|
F2 |
849 |
5.98 |
9.5 |
1.43 |
- |
849.9 |
- |
|
F3 |
853 |
5.96 |
10.0 |
1.54 |
- |
849.73 |
- |
|
F4 |
854 |
5.98 |
10.0 |
1.40 |
0.2 |
850.16 |
101.12 |
|
F5 |
851 |
6.01 |
9.5 |
1.22 |
0.1 |
850.93 |
100.34 |
|
F6 |
848 |
6.04 |
9.5 |
1.26 |
0.32 |
849.59 |
99.01 |
|
F7 |
849 |
6.06 |
9.5 |
1.26 |
0.27 |
850.3 |
103.23 |
|
F8 |
852 |
6.01 |
9.5 |
1.28 |
0.15 |
851.03 |
104.67 |
Table No. 7: Evaluation of clarithromycin coated tablets of trial batch
|
S.No. |
Formulation code |
Average Weight (mg) |
Thickness (mm)* |
Disintegration (min) |
Weight variation |
Assay (%) |
|
1 |
F4 |
874 |
6.16±0.031 |
2.24±0.019 |
874.18±1.59 |
98.87 |
|
2 |
F5 |
877 |
6.11±0.053 |
2.25±0.029 |
876.93±1.46 |
98.79 |
|
3 |
F6 |
877 |
6.17±0.064 |
2.25±0.02 |
876.09±1.35 |
103.28 |
|
4 |
F7 |
876 |
6.13±0.039 |
2.27±0.057 |
875.93±1.67 |
102.76 |
|
5 |
F8 |
874 |
6.14±0.037 |
2.25±0.046 |
877.03±0.99 |
104.54 |
Table No. 8: For optimized batches (coated tablets)
|
S. |
Form |
Average |
Thickness (mm)* |
Weight variation test* (mg) |
Disinte |
Assay# |
|
1. |
OF1 |
875.54 |
6.12±0.026 |
876.10±0.94 |
3.12±0.04 |
100.04±0.07 |
|
2. |
OF2 |
876.27 |
6.17±0.031 |
876.56±1.44 |
3.06±0.06 |
99.99±0.01 |
|
3. |
OF3 |
875.08 |
6.13±0.025 |
876.03±1.23 |
3.27±0.05 |
102.99±0.01 |
|
4. |
OF4 |
874.79 |
6.19±0.065 |
874.75±1.35 |
2.53±0.023 |
101.99±0.01 |
|
5. |
OF5 |
877.02 |
6.16±0.031 |
875.03±0.08 |
2.25±0.018 |
104.54±0.02 |
|
6. |
OF6 |
877.32 |
6.11±0.053 |
874.0±1.12 |
2.24±0.01 |
103.30±0.63 |
|
7. |
OF7 |
876.15 |
6.17±0.064 |
876.06±1.05 |
2.16±0.02 |
101.69±0.01 |
|
8. |
OF8 |
874.14 |
6.13±0.039 |
875.89±1.08 |
2.08±0.04 |
103.01±0.04 |
|
9. |
OF9 |
876.21 |
6.14±0.037 |
876.55±0.64 |
1.55±0.05 |
103.68±0.01 |
All the values are expressed as *Mean ± SD (n=6); # Mean ± SD (n=3).
COMPARATIVE DISSOLUTION PROFILE STUDIES
Table No. 9: Comparative dissolution profile study of clarithromycin coated tablets with innovator product.
|
S. |
Form |
Cumulative percentage Drug release (min)* |
||||
|
5th min |
10th min |
15th min |
20th min |
30th min |
||
|
1. |
F4 |
68.55±0.12 |
81.57±0.72 |
91.23±0.85 |
92.67±0.23 |
96.34±0.12 |
|
2. |
F5 |
68.84±0.45 |
84.32±0.75 |
91.75±0.12 |
93.62±0.83 |
98.38±0.34 |
|
3. |
F6 |
71.04±0.64 |
80.56±0.53 |
91.89±0.64 |
95.33±0.87 |
99.47±0.46 |
|
4. |
F7 |
69.38±0.87 |
82.74±0.65 |
93.40±0.25 |
96.65±0.37 |
102.11±0.47 |
|
5. |
F8 |
69.19±0.16 |
85.31±0.25 |
96.27±0.76 |
98.36±0.65 |
101.15±0.72 |
|
6. |
Inno |
80.88±0.04 |
95.46±0.14 |
97.55±0.41 |
99.34±0.23 |
102.56±0.34 |
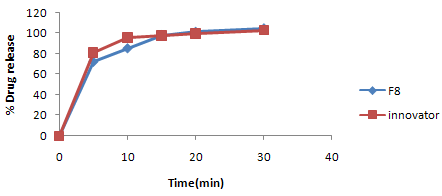
Figure No. 2: Comparative dissolution profile study of clarithromycin coated tablet (F8) with innovator product
Table No. 10: Comparative dissolution profile study of clarithromycin optimized coated tablets with innovator product
|
S.No. |
Formulation Code |
Cumulative percentage Drug release(min)* |
||||
|
5th min |
10th min |
15th min |
20th min |
30th min |
||
|
1. |
OF1 |
70.28 |
83.61 |
95.91 |
97.56 |
99.38 |
|
2. |
OF2 |
69.04 |
83.19 |
94.67 |
96.73 |
98.21 |
|
3. |
OF3 |
68.65 |
82.59 |
94.76 |
96.91 |
98.57 |
|
4. |
OF4 |
70.59 |
84.68 |
95.03 |
98.21 |
100.46 |
|
5. |
OF5 |
69.19 |
83.31 |
95.27 |
97.36 |
101.15 |
|
6. |
OF6 |
68.59 |
82.11 |
94.98 |
96.02 |
99.83 |
|
7. |
OF7 |
70.83 |
84.96 |
95.45 |
98.46 |
101.62 |
|
8. |
OF8 |
69.54 |
83.63 |
94.86 |
96.08 |
100.28 |
|
9. |
OF9 |
68.73 |
82.77 |
93.88 |
96.46 |
101.14 |
|
10. |
Innovator |
80.88 |
95.46 |
97.55 |
99.34 |
102.56 |
All the values are expressed as * Mean SD (n=6).
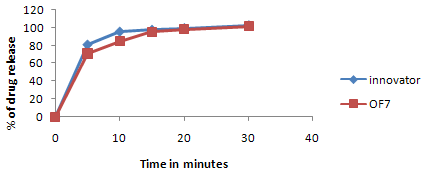
Figure No.3:Comparative dissolution profile study of clarithromycin optimized coated tablet (OF7) with innovator product
Table No. 11: Comparative dissolution profile (Dissimilarity factors (f1) and Similarity factor (f2)) for OF7 and innovator product.
|
S. No. |
Dissolution time points |
Formulation OF7* |
Innovator product* |
Formulation OF7 |
|
|
Dissimilarity factors (f1) 0-15 |
Similarity factor (f2) 50-100 |
||||
|
1. |
5th min |
70.83 |
80.88 |
5.147 |
59.658 |
|
2. |
10th min |
84.96 |
95.46 |
||
|
3. |
15th min |
95.45 |
97.55 |
||
|
4. |
20th min |
98.46 |
99.34 |
||
|
5. |
30th min |
101.62 |
102.56 |
||
All the values are expressed as *Mean ± SD (n=6).
STABILITY STUDIES
Table No. 12: Stability study data for OF7 formulation (Post compression parameters)
|
Post compression |
Storage condition 40?C ± 2?C / 75% RH ± 5% RH |
|||
|
Initial |
1st month |
2nd month |
3rd month |
|
|
Description |
* |
* |
* |
* |
|
Average weight (mg) |
875.0±0.85 |
875.0±0.96 |
876.0±0.05 |
876.15±0.02 |
|
Hardness(Kg/cm2) |
10.0±0.09 |
9.5±0.77 |
9.5±0.44 |
9.5±0.09 |
|
Thickness(mm) |
6.17±0.037 |
6.17±0.12 |
6.16±0.34 |
6.16±0.02 |
|
Disintegration time (sec) |
2.16±0.046 |
2.20±0.06 |
2.14±0.08 |
2.06±0.05 |
* yellow coloured film-coated tablet.
Table No. 13: In vitro dissolution study –OF7, stability batch formulation
|
Dissolution Time points (min) |
Storage condition 40?C ± 2?C / 75% RH ± 5% RH |
||||
|
Initial |
1st month |
2nd month |
3rd month |
Innovator |
|
|
5 |
70.83±0.354 |
69.45±0.21 |
65.27±0.70 |
61.66±0.28 |
77.34±0.18 |
|
10 |
84.96±0.57 |
84.23±0.54 |
83.65±0.69 |
81.57±0.42 |
93.67±0.22 |
|
15 |
95.45±0.54 |
94.89±0.16 |
93.45±0.21 |
92.03±0.27 |
95.27±0.76 |
|
20 |
98.46±0.66 |
98.24±0.02 |
97.82±0.25 |
97.56±0.92 |
98.87±0.01 |
|
30 |
101.62±0.48 |
101.03±0.06 |
100.67±0.62 |
99.73±0.12 |
100.56±0.05 |
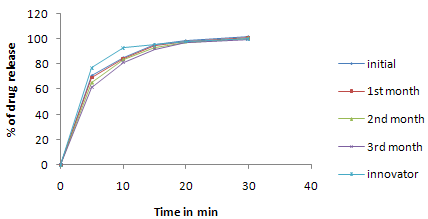
Figure No. 4: In vitro dissolution study –OF7, stability batch formulation
Table No. 14: Assay – OF7, stability batch formulation
|
Assay (%) |
Storage condition 40?C ± 2?C / 75% RH ± 5% RH |
|||
|
Initial |
1st month |
2nd month |
3rd month |
|
|
OF7 |
101.69 |
100.27 |
99.74 |
96.88 |
|
Innovator |
99.34 |
98.70 |
97.62 |
96.47 |
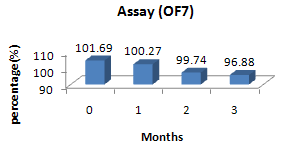
Figure No. 5:Assay – OF7, stability batch formulation.
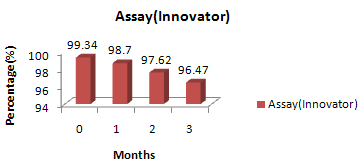
Figure No. 5:Assay – Innovator, stability batch formulation.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CHROMATOGRAM OF CLARITHROMYCIN TABLETS
Table No. 15: Chromatogram of clarithromycin and their related substance
|
S.No. |
Name |
Retention time(min) |
Area(AU) |
Area (%) |
Tailing factor |
|
1 |
Clarithromycin standard |
8.845 |
338781 |
40.81 |
1.99 |
|
2 |
Clarithromycin related compound A |
12.812 |
491391 |
59.19 |
2.13 |
Figure No. 6:Chromatogram of clarithromycin and their related substance
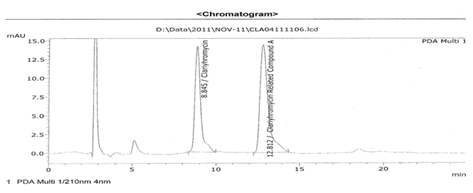
Table No. 16: Clarithromycin (OF7) stability batch chromatograms (dissolution profile)
|
S.No. |
Name of the sample |
Retention time (min) |
Area (AU) |
Area (%) |
Tailing factor |
|
1 |
Standard |
8.955 |
338875 |
100.00 |
1.306 |
|
2 |
OF7 3rd month |
8.950 |
301055 |
100.00 |
1.30 |
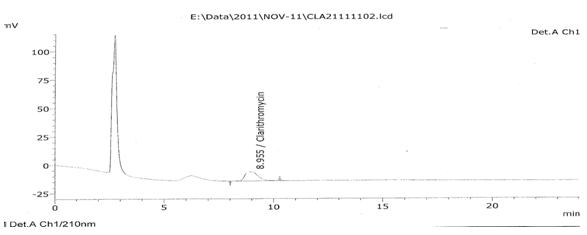
Figure No. 7:Chromatogram of clarithromycin standard (Standard)
Figure No. 13: Chromatogram of clarithromycin tablets (40oC ±2%,75%±5% RH)-3rd month
Table No. 17: Clarithromycin (OF7) stability batch chromatograms (Assay)
|
S.No. |
Name of the sample |
Retention time (min) |
Area(AU) |
Area (%) |
Tailing factor |
|
1 |
Standard |
8.956 |
338875 |
100.00 |
1.38 |
|
2 |
OF7 3rd month |
8.954 |
328283 |
100.00 |
1.38 |
Figure No. 8:Chromatogram of assay for clarithromycin (Standard)
Figure No. 9:Chromatogram of assay of clarithromycin tablets
DISCUSSION
The present study of clarithromycin film coated tablets were developed with a view to deliver the drug immediately. The film coated immediate release tablets were evaluated and the details of results and discussion were given in the following sections.
Drug Excipient-Compatibility Study:
The FT-IR spectrum of the combined clarithromycin and excipients was shown in the Fig.No 1. The spectrum shows the presence of peaks at 3465 cm-1 ,2975 cm-1,1730 cm-1 , 1459 cm-1 ,1228 cm-1 ,1026 cm-1 and 1011 cm-1of OH,CH, C=O, CH3,CH2, C-N, C—O stretching respectively, indicating there is no interation between the drug and the excipients.
Observation of clarithromycin tablet formulation during inprocess:
Initial batches, that is F1 to F3 were formulated with wet granulation by aqueous method with hydroxy propyl cellulose (2.35%) as a binder, MCC PH 101 is used as a diluent. These formulations shown a sticking problem during inprocess compression, which may be due to high moisture content, low binding and low amount of lubricants.
Therefore, the next trial (F4) were again formulated with non-aqueous granulation with povidone (4.029%) and lubricants like aerosil (0.529%), magnesium stearate (1.0%), talc (0.5%). In this trial, sticking was not observed. But roughness is observed during compression.
In the trial F5, in this formulation, MCC PH 101 is replaced by MCC PH 102 (4.23%) and colloidal silicon dioxide (0.7%) in the granulation. In addition, lubricants are increased to avoid the sticking problem during compression. But roughness is observed during compression.
In the F6 trial, some amounts of lubricants are increased in both upper and lower granulation parts. All the parameters were found to be satisfactory and this batch tablets were kept for stability studies. During stability studies, dissolution was failed in the 1st month for 40oC ± 2oC/75% ± 5%. Because, while dissolution, the tablet breaks into 2-3 parts and not disintegrated uniformly. The percentage drug release was also less compared to initial month of stability studies. This problem may be due to insufficient disintegrates in the formulation.
In the trial F7, this procedure is also same as formulation trial F5. However, in this formulation, the concentration of MCC PH102 is decreased and pre-gelatinized starch was included in the lubrication part of the formulation for better disintegration during the dissolution. In this, all the parameters were found satisfactory during pre-compression and post-compression. During dissolution of 1st month of stability studies, all the tablets were not disintegrated evenly and divided in to 2 to 3 parts. This may be due to presence of aerosil in the upper granulation part.
In the trial F8, in this formulation, colloidal silicon dioxide was replaced from granulation part to the lubrication part. In addition, increase the lubricants concentration to avoid sticking. In this trial, all the parameters were found satisfactory at initial stages.
Therefore, to know the best formula, formulation F8 undergoes the 32 randomized full factorial optimization studies. Based on preformulation studies, the amounts of croscarmellose sodium (X1) and microcrystalline cellulose PH102 (X2) were selected as the independent factors, studied at 3 levels each (-1, 0, +1). The percentage drug release (y1) and disintegration time (y2) were taken as dependent factors.
Optimized batches were coded as OF1, OF2 OF3, OF4, OF5, OF6, OF7, OF8 and OF9. The Precompression and post compression studies was performed for all the optimized batches. Results were found to be similar for all the optimized batches and innovator product. From these studies, OF7 was selected and compared all the evaluation profiles with the innovator product during the period of stability studies.
Evaluation of blend materials of clarithromycin tablets:
The angle of repose of formulation blends of clarithromycin F1 to F8 were in the range of 30.14±0.29° to 34.93±0.68°. The bulk density, tapped density, Carr’s index, hausners ratio were found in the range of 0.472 to 0.534g/cc, 0.55 to 0.61g/cc, 10 – 15.33g/cc and 1.11-1.18 respectively. It reveals that all the formulation blends were having good flow characteristics and flow rates.
The results of granule evaluation were given in Table No. 5.
Tablet characteristics of clarithromycin uncoated IR tablets:
The tablets of different formulation were subjected to various evaluation tests such as thickness, hardness, friability and drug content. All the formulations of clarithromycin showed uniform thickness.
The hardness and percentage friability of all batches (F4 to F8) of clarithromycin ranged from 9.5 – 10.0 kg / cm2 and 0.1 – 0.3 % respectively. The disintegration of all batches (F4 to F8) of clarithromycin is found in limits 1.22-1.54.
The drug content of clarithromycin uncoated tablets was found to be uniform among all the formulations which ranges from 99.01% –104.67%. The evaluation results of clarithromycin uncoated IR tablet were given in Table No.6
Tablet characteristics of clarithromycin coated IR tablet:
The tablets of different formulation were subjected to various evaluation tests such as thickness, disintegration and drug content. All the formulations of clarithromycin showed uniform thickness.
The disintegration time of all batches (F4 to F8) of clarithromycin is found within limits 2.24-2.27.
The drug content of clarithromycin coated tablets was found to be uniform among all the formulations, which ranges from 98.87% – 104.54%. The evaluation results of clarithromycin IR tablet were given in Table No.7
Tablet characteristics of clarithromycin optimized coated IR tablet:
The tablets of different formulation were subjected to various evaluation tests such as thickness, disintegration and drug content.
The disintegration of all batches (OF1 to OF9) of clarithromycin are found within limits 2.08-3.06 min.
The drug content of clarithromycin coated tablets was found to be uniform among all the formulations which ranges from 99.99% – 104.54%. The evaluation results of clarithromycin IR tablet were given in Table No.8.
In-vitro drug release study from F4to F8
The in vitro drug release of all the formulations of clarithromycin from F4 to F8 at 5th, 10th, 15th, 20th and 30th minutes was found to be in the range of 68.55-69.19%, 81.57-85.31%, 91.23-96.27%, 92.67-98.36%, and 96.34-101.15% respectively. Among all the formulations, F8 were found to be the best (F8-Clarithromycin-500mg, CCS-17 mg, povidone - 35mg, CCS (L) - 25.50mg, MCC102-196mg, pregelatinized starch (L)-50mg, talc - 9.50 mg, aerosil l-9.50 %, magnesium stereate-7.50mg) since its release was satisfactory i.e., 69.19%,85.31%, 96.27%,98.36%, 101.15% at 5th,10th,15th,20th,30th minute. The results were decipieted in Table No.9.
Comparison of clarithromycin IR tablets (OF7) with innovator product
Table No.10, gives the comparison of in-vitrodissolution profile of clarithromycin IR batch (OF7) with the innovator product. The drug release of clarithromycin IR tablet was found to be 70.83%, 84.96%, 95.45%, 98.46%, and 101.62% at 5th, 10th, 15th, 20th, 30th min respectively.
The drug release of innovator product was found to be 80.88%, 95.46%, 97.55%, 99.34% and 102.56% at 5th,10th,15th,20th ,30th minute respectively for clarithromycin .
In Table No.11, the formulation OF7shows the dissimilarity factor f1 and similarity factor f2 values are within the specified limits (i.e., 5.147 and 59.658) when compared with the innovator product. Hence, formulation OF7 was selected for stability studies.
Stability Studies
The clarithromycin immediate release tablets (OF7) was kept on stability at 40° C/ 75 % RH and the three month accelerated condition results were found to be satisfactory. The stability study data’s were depicted in the Table No. 12-17.
CONCLUSION
The objective of the present study was to formulate, optimize and evaluate clarithromycin immediate release film coated tablet.
Literatures regarding, clarithromycin tablet dosage form preparation, excipients selection, manufacturing method, etc., has been collected and reviewed.
In this work, selection of excipient was done based on standard innovator product cited from pack insert. Excipients include croscarmellose sodium, povidone, pregelatinized starch, microcrystalline cellulose, colloidal silicon dioxide, magnesium stearate.
The tablets were formulated by wet granulation method using the selected excipient quantities. The formulated tablets were tested for both pre-compression parameters, post compression parameters as per requirements of standards performed and found to be within the limits.
The formulated trial batch was taken for optimization by full factorial design. i.e., croscarmellose sodium (X1) and microcrystalline cellulose sodium (X2) as 2 independent variables at 3 levels -1, 0 and +1.
Optimized batches were coded as OF1, OF2, OF3, OF4, OF5, OF6, OF7, OF8 and OF9. The in vitro dissolution study was performed for all the optimized formulations. Similarity is found in the results of all the optimized formulations and innovator product.
During the optimization of formulation it was observed in dissolution that decreasing the concentration of diluent, increasing release profile could be achieved by increasing the concentration of disintegrant. Simultaneously, pre gelatinized starch also increased by decreasing the concentration of diluent in OF7 formulation.
Among the entire optimized batches, formulation OF7 has been selected for calculating similarity factor, since it shows better results (i.e., faster disintegration time and rapid drug release) than other optimized batches. Similarity factor was calculated by comparing the in-vitro drug release profile for batch OF7 with the innovator product. The dissimilarity factor f1 value of 5.147 and similarity factor f2value of 59.658 indicates that the two products were similar in in-vitro drug release.
The tablets of OF7optimized batch was subjected to accelerated stability studies as per ICH guidelines. The results of stability studies showed that there were no significant changes in the physical and chemical parameters studied.
From this study, it was concluded that optimized clarithromycin tablet (OF7) containing croscarmellose sodium (3.029%) and pregelatinized starch (6.029%)could be manufactured with reproducible characteristics from batch to batch.
ACKNOWLEDGEMENT:
Author thank to Head and Guideof Department of Pharmaceutics, Sankaralingam Bhuvaneswari College of Pharmacyfor providing necessary facilities to carry out this study.
REFERENCES:
1. Goodman and Gilman’s manual of pharmacology and therapeutics. Laurence Bruton, Keith parker, Donald Blumenthal and Lain Buxton: 11th Edition, United states, McGraw hill companies, 2008; 769-773.
2. Lippincott’s illustrated reviews: pharmacology. Lippincott’s Williams and wilkins: 4th edition, 2009; 379-382.
3. Rang and dale’s pharmacology. H.P. Rang, M.M. Dale, J.M. Ritter and R.J.Flower: 6th Edition. 666-672.
4. Banker GS, Anderson NR. Tablets. Lachman L, Lieberman HS, Kanig JL: The Theory and Practice of Industrial Pharmacy. Varghese Publishing House : 296-301.
5. Indian Pharmacopoeia. Ministry of health and family welfare, Govt. of India, controller of publications, 2010. New Delhi.
6. Clarithromycin tablets, Unied States Pharmacopiea 30-National Formulary 25.2007; 1771.
7. Indian Pharmacopoeia, Ministry of health and family welfare, Govt. of India, controller of publications, New Delhi: 1996; 736: A80-83.
8. Rai VK., Pathak N., Bhaskar R., Nandi BC., Dey S. and Tyagi LK: Optimization of immediate release tablet of Raloxifene Hydrochloride by wet granulation method. Int J Pharm Sci Drug research. 2009; 1(1) : 51-54.
9. Rabia B., Muhammad HS., Nousheen A., Durriya H. and Masud-Ur-Rehman: Formulation development and optimization of ibuprofen tablets by direct compression method. Pak J Pharm Science 2008; 21(2): 113-120.
10. Comparison of dissolution profiles using similarity factors. [Online]. Available at: pharmainfo.net/Dissolution/comparison-dissolution-profiles-using-f1-and-f2-factors.
11. Mukesh CG., Krishnakant GS., Neelima RM., Chirag DS., Vinita UV. and Rikita KD: Assessment of similarity factor using different weighting approaches. Dissolution technologies. 2005; 22-27.
12. Ma M., Lin R. and Liu J: Statistical evaluations of dissolution similarity. Statistica Sinica, 1999; 9 : 1011-1027.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









