{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Kambham Venkateswarlu*,
Department of Pharmaceutics,
JNTUA-Oil Technological and Pharmaceutical Research Institute
Andhra Pradesh, India
k.v.reddy9441701016@gmail.com
ABSTRACT
The objective of the present investigation was to formulate and evaluate the mouth dissolving tablets (MDTs) of ketoprofen (KPN) using disintegrants like Crospovidone (CP), Croscarmellose sodium (CCS) and Sodium starch glycolate (SSG) and the effect of disintegrants on drug release was also studied. The powder blend was evaluated for flow properties and found acceptable flow properties. The prepared MDTs were evaluated for post compression parameters such as hardness, friability, weight variation, dispersion time, drug content and in-vitro drug release. Tablets possessing sufficient strength supported by hardness and friability results. The formulation KP6 showed lease dispersion time of 14 sec and it was selected for drug release studies. KP6 showed 99.9% of drug release in 5 min and time taken for 70% of the drug is 2.8 min. Based on the results obtained from the dispersion time and in-vitro dissolution studies, it could be concluded that the concentration of disintegrant is indirectly proportional to the dispersion time and directly proportional to the amount of drug release respectively.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2499
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 6 Received On: 21/02/2017; Accepted On: 16/03/2017; Published On: 01/06/2017 How to cite this article: Venkateswarlu K;Formulation and in-vitro valuation of Mouth Dissolving Tablets of Ketoprofen: Effect of Disintegrants on drug release; PharmaTutor; 2017; 5(6); 17-21 |
INTRODUCTION
Solid dosage forms like tablets and capsules are most popular and preferred drug delivery systems because they have high patient compliance, relatively easy to produce, easy to market, accurate dosing, good physical and chemical stability.[1] Oral drug delivery has been known for decades as the most widely utilized route of administration among all the routes that have been explored for the systemic delivery of drugs via various pharmaceutical products of different dosage forms and the reason might be due to the easy administration, self-medication and patient compliance. From past decade, there has been an increased demand for more patient-friendly and compliant dosage forms. As a result, the demand for developing new technologies has been increasing day by day. [2] KPN comes under the category of NSAIDs and it possesses good analgesic activity. The main drawback of KPN is its low water solubility which can cause bioavailability problems. There are many techniques currently using for improving the solubility of poorly soluble drugs such as solid dispersions, salt formation and complexation with Cyclodextrin etc.[3] The preparation of tablets using above techniques may be costly and time taking. Hence, in the present study, the step was taken to prepare the KPN mouth dissolving tablets by direct compression method. The solubility of KPN was improved by using different types of super disintegrants. The direct compression method is an easy and cheap method when compared to other techniques and it may expect that the time saving will be more.
Materials and methods
Materials
KPN was obtained from Cipla Pharma Ltd., India. Superdisintegrants like CCS, CP and SSG were obtained from HiMedia Laboratories Ltd., India. Remaining ingredients/chemicals used were of analytical grade and obtained from Merck Specialties Ltd., India.
Preparation of KPN tablets
All the ingredients mentioned in table 1 were passed through 60 mesh sieve separately. Then the drug and diluents were taken separately a small portion at each time and blended it thoroughly to get a uniform mixture. The obtained mixture was compressed by Rimek mini press machine using 8 mm size punch.[4]
FTIR studies
Drug excipients compatibility studies were performed by FTIR spectroscopy. KBr disc method was used for the preparation of samples and those discs were scanned from 4000 to 400 cm-1. The obtained spectrum of the pure drug was compared with the spectrum of its physical mixture for identifying changes in the position of the characteristic peaks.[5]
Flow property studies
The powder blend was subjected to flowability tests such as Carr’s index (CI), Hausner’s ratio (HR), angle of repose (AR) and porosity described by Venkateswarlu et al.[6-7]
Evaluation of tablets
MDTs were evaluated for hardness, friability [6,8], weight variation [6,9] and in-vitro dispersion time.[6, 10]
Drug content uniformity
Twenty tablets were crushed in a mortar and weighed the powder equivalent to 100 mg of drug transferred into a conical flask containing pH 1.2 HCl buffer. The suitable dilutions were done and measured at 296 nm using UV-Visible spectrophotometer (Shimadzu, Japan).[6,11]
In-vitro dissolution studies
Dissolution rate was studied by USP type-II dissolution apparatus (Lab India, India) using 900 ml of pH 1.2 HCl as dissolution medium. The temperature and rpm were maintained at 37±0.5°C and 50 respectively. The aliquot of dissolution medium was withdrawn at every 1 min interval, filtered and absorbance was measured using UV-Visible spectrophotometer at 296 nm. [6,12-14]
Table 1. Composition of KPN MDTs
|
Ingredient (mg) |
KP1 |
KP2 |
KP3 |
KP4 |
KP5 |
KP6 |
KP7 |
KP8 |
KP9 |
KP10 |
KP11 |
KP12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
KPN |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
|
CP |
1.6 |
4.8 |
8.0 |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
CCS |
-- |
-- |
-- |
3.2 |
6.4 |
8.0 |
-- |
-- |
-- |
1.6 |
3.2 |
4.8 |
|
SSG |
-- |
-- |
-- |
-- |
-- |
-- |
6.4 |
9.6 |
12.8 |
1.6 |
3.2 |
4.8 |
|
Microcrystalline cellulose |
52 |
48.8 |
45.6 |
50.6 |
47.2 |
45.6 |
47.2 |
44.8 |
40.8 |
50.4 |
47.2 |
44 |
|
Lactose |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
|
Talc |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
|
Mg stearate |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
3.2 |
|
Total |
210 |
210 |
210 |
210 |
210 |
210 |
210 |
210 |
210 |
210 |
210 |
210 |
Results and discussion
MDTs of KPN were prepared by direct compression method using super disintegrants like CCS, CP and SSG alone and in combination. The probability/possibility of drug-polymer interaction was studied by Infrared Spectroscopy using KBr pellet method. The Infrared Spectra of the pure drug KPN showed its characteristic absorption peaks at the wave numbers 2957.79, 1667.24 and 1422 cm-1 corresponding to C-H str, C=O str and C=N str respectively. When the spectrum of the formulation compared with the spectrum of pure KPN, the characteristic peaks of pure drug were retained indicating the intactness of the drug in formulation prepared. All the powder blends of formulations prepared were evaluated for precompression parameters like CI, HR, AR and Percentage porosity and are given in table 2. The compressibility index values less than 10, 11-15, 16-20, 21-25, 26-31, 32-37 and greater than 38 indicates excellent, good, fair, possible, poor, very poor and very very poor flow respectively and powder blends of all the formulations were found to be possessing the excellent to good flowability i.e. 10.52 to 16.53%. The ideal Hausner’s ratio values of 1.0-1.11, 1.12-1.18, 1.19-1.25, 1.26-1.34, 1.35-1.45, 1.46-1.59 and greater 1.60 indicates excellent, good, fair, possible, poor, very poor and very very poor flow respectively and powder blends of all the formulations were found to be possessing the excellent to fair flowability i.e. 1.11 to 1.19. The ideal angle of repose values of less than 25, 25-30, 30-40 and greater than 40 indicates excellent, good, passable and very poor respectively and the experimental values of all the formulations were ranged from 26.28 to 31.03ͦ° indicates good to passable flowability.[6, 15] Porosity was ranged from 56.1 to 57.4%. Fast dissolving tablets so prepared were evaluated for post-compression parameters like hardness, friability, drug content uniformity, weight variation, dispersion time, in-vitro drug release studies and results were represented in table 3-4. Hardness of the three tablets of each batch was checked using Monsanto hardness tester and ranged from 2.85 to 4.01 kg/cm2. Friability was determined by Roche friabilator and variable from 0.53 to 0.86 % and It complies with the official limits (<1%). The results from hardness and friability studies indicated that the tablets possessing sufficient strength and can withstand to the mechanical breakdowns during shipping etc. Drug content was ranged from 95-100% and complied with the standards. Weight variation studies proved that all the tablets possessing the uniform weight (358-363 mg) and followed the official limits (±10%).[6,15] Formulation KP6 showed the least disintegration of 14 sec and it was selected for further studies. In-vitro dissolution studies showed the drug release of 99.91% in 5 min and time taken to release 70% of the drug was found to be 2.8 min.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
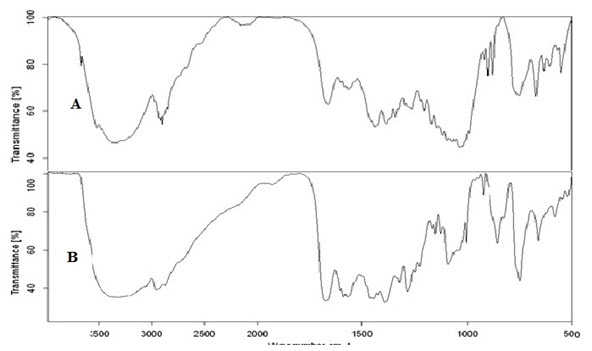
FTIR studies. A) IR spectrum of physical mixture, B) IR spectrum of pure drug
Table 2. Physical properties of powder blend
|
Formulation |
CI (%) |
HR
|
AR (°) |
Percentage Porosity |
|---|---|---|---|---|
|
KP1 |
11.53±0.10 |
1.11±0.56 |
28.32±0.13 |
56.1±0.96 |
|
KP2 |
13.52±0.35 |
1.15±0.61 |
29.08±0.18 |
57.3±0.91 |
|
KP3 |
15.55±0.68 |
1.17±0.48 |
30.21±0.69 |
56.2±0.67 |
|
KP4 |
14.94±0.43 |
1.16±0.39 |
30.11±0.67 |
56.5±0.34 |
|
KP5 |
13.81±0.98 |
1.14±0.91 |
28.43±0.51 |
57.4±0.95 |
|
KP6 |
15.10±0.47 |
1.18±0.97 |
30.38±0.59 |
56.8±0.84 |
|
KP7 |
16.53±0.35 |
1.19±0.63 |
31.03±0.98 |
56.9±0.73 |
|
KP8 |
12.74±0.84 |
1.12±0.58 |
28.10±0.0.64 |
56.7±0.38 |
|
KP9 |
10.52±0.65 |
1.15±0.68 |
26.28±0.34 |
56.4±0.71 |
|
KP10 |
14.61±0.19 |
1.17±0.34 |
29.78±0.65 |
56.2±0.78 |
|
KP11 |
10.99±0.14 |
1.16±0.28 |
26.89±0.97 |
56.3±0.63 |
|
KP12 |
11.35±0.61 |
1.12±0.53 |
26.45±0.35 |
56.8±0.16 |
Values were expressed in mean±SD
Table 3. Post compression studies of tablets
|
Formulation |
Hardness (kg/cm2) |
Friability (%) |
Dispersion Time (sec) |
Weight Variation (mg) |
Drug content |
|---|---|---|---|---|---|
|
KP1 |
3.23±0.12 |
0.53±0.56 |
76±2.50 |
358±0.98-363±110 |
99.5 ±0.17 |
|
KP2 |
3.24±0.28 |
0.53±0.35 |
61±1.41 |
100.0 ±0.27 |
|
|
KP3 |
3.35±0.12 |
0.56±0.65 |
39±1.83 |
97.0 ±0.17 |
|
|
KP4 |
3.24±0.24 |
0.61±0.68 |
58±0.74 |
96.0 ±0.43 |
|
|
KP5 |
2.91±0.36 |
0.63±0.98 |
38±0.48 |
99.3 ±0.35 |
|
|
KP6 |
2.85±0.32 |
0.58±0.64 |
14±0.50 |
99.0 ±0.15 |
|
|
KP7 |
3.18±0.12 |
0.59±0.54 |
109±1.19 |
95.5 ±0.26 |
|
|
KP8 |
2.92±0.01 |
0.63±0.18 |
85±1.40 |
99.5 ±0.24 |
|
|
KP9 |
2.96±0.14 |
0.69±0.31 |
68±0.76 |
97.0 ±0.15 |
|
|
KP10 |
4.01±0.00 |
0.86±0.84 |
86±0.94 |
96.0 ±0.12 |
|
|
KP11 |
3.71±0.12 |
0.77±0.74 |
64±0.71 |
99.3 ±0.43 |
|
|
KP12 |
3.71±0.28 |
0.55±0.78 |
45±0.68 |
96.5 ±0.14 |
Values were expressed in mean±SD
Table 4. Dissolution profile of KP6 and its T70%
|
Time (min) |
*KP6 |
T70% (min) |
|
01 |
25.92 ±0.44 |
2.8 |
|
02 |
53.12 ±0.46 |
|
|
03 |
74.62 ±0.12 |
|
|
04 |
92.94 ±0.11 |
|
|
05 |
99.91 ±0.01 |
Values were expressed in mean±SD
Effect of disintegrants on drug release
As per official standards [6,16], MDTs or orally disintegrating tablets should disintegrate within 1 min and it was satisfied by the formulations such as KP3, KP4, KP5, KP6 and KP12. In the present study, the effect of disintegrants alone and in combination on drug release was studied. It was observed from the results that the KP6 showed least dispersion time of 14 sec and formulations like KP1, KP2 and KP7-11 were failed to dispersion test. When compared to the disintegrating agents, CCS alone showed a greater effect on dispersion of tablets thereby it releases the drug rapidly and SSG showed least dispersion property. CP showed medium level dispersion property compared to the CCS and SSG. The order of effectiveness (alone): CCS>CP>SSG. In a combination of disintegrants SSG and CCS couldn’t show the better dispersion results than in alone usage of CCS. It was confirmed from the results, the increase in the concentration of disintegrant showed decrease in dispersion time i.e. concentration of disintegrant is indirectly proportional to the dispersion time.
CONCLUSION
The MDTs of KPN were successfully prepared by direct compression method. It could be concluded from the results that the concentration and type of disintegrant used will play a crucial role in drug release from its dosage form. It is also observed that the concentration of disintegrant is indirectly proportional to the dispersion time or it can also say that the increase in the concentration of disintegrant cause increase in drug release rate.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONFLICT OF INTEREST
The authors report no conflict of interests. The author is responsible for content and writing of the paper.
REFERENCES
1. Venkateswarlu K, Preethi JK, Chandrasekhar KB. Formulation and in-vitro evaluation of loperamide immediate release tablets by liquisolid technique. Adv. Pharm. Bull. 2016; 6(3): 385-90.
2. Venkateswarlu K, Chandrasekhar KB. Development of stavudine sustained release tablets: in-vitro studies. Future J. Pharm. Sci. 2016; 2(2): 37-42.
3. Vittal GV, Deveswaran R, Bharath S, Basavaraj BV, Madhavan V. Formulation and characterization of ketoprofen liquisolid compacts by Box-Behnken design. Int. J. Pharm. Investig. 2012;2:150-6.
4. Venkateswarlu K, Chandrasekhar KB. Development and Statistical Optimization of Sustained Release Gastro Retentive Floating Tablets of Cephalexin. Marmara Pharm. J. 2016; 22(2): 172-183.
5. Vijayabhaskar K, Venkateswarlu K, Thirumalesh Naik SB, Kiran Jyothi R, Nethra Vani G, Chandrasekhar KB. Preparation and in-vitro Evaluation of Ranitidine Mucoadhesive Microspheres for Prolonged Gastric Retention. Br. J. Pharm. Res. 2016; 10(2): 1-12.
6. Asian Rockville MD, Editor. United States Pharmacopoeia 24/NF19. The Official Compendia of Standards. United States Pharmacopoeia Convention Inc; 2000.
7. Venkateswarlu K, Chandrasekhar KB, Ramachandra R. Development and in-vitro Evaluation of Reconstitutable Suspension of Flucloxacillin. Marmara Pharm. J. 2016; 20(3): 280-7.
8. Thirumalesh Naik SB, Venkateswarlu K, Chandrasekhar KB. Formulation and in-vitro evaluation of Pregabalin mini tablets for sustained release. Pharm. Lett. 2016; 8(2): 277-83.
9.Venkateswarlu K, Shanthi A. Formulation and evaluation of sustained release Glipizide matrix. IOSR J. Pharm. Biol. Sci. 2012; 2(5): 17-23.
10. Thirumalesh Naik SB, Venkateswarlu K, Chandrasekhar KB. Formulation and evaluation of Oxybutynin chloride extended release matrix tablets. IndoAm. J. Pharm. Res. 2016; 6(01): 4179-84.
11. Kuchekar BS, Badhan AC, Mahajan HS. Oral dissolving tablets of salbutamol sulphate: A novel drug delivery system. Indian Drugs. 2004; 41: 592-8.
12. Venkateswarlu K, Thirumalesh Naik SB, Chandrasekhar KB. Formulation and in vitro evaluation of orlistat orodispersible tablets for enhancement of dissolution rate. Int. J. Pharm. Pharm. Sci. 2016; 8(4): 236-241.
13. Venkateswarlu K, Preethi JK, Kiran BSS. Formulation Development and In-vitro Evaluation of Floating Tablets of Ciprofloxacin HCl. Asian J. Pharm. 2016; 10(4): 271-278.
14.Venkateswarlu K, Preethi JK. Formulation development and in vitro evaluation of mouth dissolving tablets of pioglitazone hydrochloride. Pharmatutor. 2016; 4(12): 37-42.
15.Venkateswarlu K, Nirosha M, Kishore Kumar Reddy B, Heerasingh T, Manasa S. Formulation and In-vitro Evaluation of Quetiapine Fumarate Extended Release Tablets using Natural Polymers. Lat. Am. J. Pharm. 2017; 36(2): 392-398.
16.Thirumalesh Naik SB, Venkateswarlu K, Chandrasekhar KB. Formulation and in-vitro evaluation of orodispersible tablets of olanzapine for the improvement of dissolution rate. J. Chem. Pharm. Res. 2016; 8(1): 177-181.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









