{ DOWNLOAD AS PDF }
ABOUT AUTHORS
P. S. Bhandare 1, V.G Gharge 2.
1*Department of Pharmacology,
GIPER, Satara, Maharashtra, India.
2 Department of Pharmaceutics,
GIPER, Satara, Maharashtra, India.
ABSTRACT
"The objective of the present investigation was to formulate and evaluate microencapsulated Glimepiride produced by the emulsion - solvent evaporation method. Microparticles were prepared using Eudragit RLPO by emulsion solvent evaporation and characterized for their micromeritic properties, encapsulation efficiency, particle size, In vitro release studies were performed in phosphate buffer (pH 7.4). The resulting microparticles obtained by solvent evaporation method were free flowinging nature. The mean particle size of microparticles ranges from 140.40 - 173.90 μm and encapsulation efficiency ranges from 90.46 – 93.09%. Eudragit RLPO microparticles containing Glimepiride could be prepared successfully by using an emulsion solvent evaporation technique, which will not only sustain the release of drug but also manage complicacy of the diabetes in a better manner.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2601
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 8 Received On: 26/06/2018; Accepted On: 18/07/2018; Published On: 01/08/2018 How to cite this article: Gharge, V.G. and Bhandare, P.S. 2018. Formulation and evaluation of microencapsulated Glimepiride produced by the emulsion - solvent evaporation method. PharmaTutor. 6, 8 (Aug. 2018), 27-30. DOI:https://doi.org/10.29161/PT.v6.i8.2018.27 |
INTRODUCTION:
Glimepiride (Sahoo 2005) is the only third generation sulphonyl urea, which lowers the blood glucose level in the healthy subjects as well as in patients with type II diabetes. After oral administration, Glimepiride is completely (100%) absorbed from the GI tract. Studies with single oral doses in normal subjects and with multiple oral doses in patients with type II diabetes have shown significant absorption of Glimepiride within 1 hour after administration and peak drug levels (Cmax) at 2 to 3 hours. Due to its low biological half-life, it requires frequent administration to maintain plasma concentration. This leads fluctuations in plasma concentration and also causes inconvenience to the patient. Therefore, development of controlled release dosage forms would clearly be beneficial in terms of decreased dosage requirements, thus increase patient compliance. Microencapsulation is a well-known method for the preparation of microparticles for controlled release. Among the various methods developed for formulation of microparticles, solvent evaporation method is one of the mostly widely used one to formulate Microparticles because of its ease of fabrication without compromising the activity of drug. In the present investigation Eudragit RLPO is used as a rate retardant polymer. Eudragit RLPO is water insoluble polymer which is widely used as a wall material for controlled release microparticles. This is due to its biocompatibility, good stability, easy fabrication and low cost. In the present Investigation solvent evaporation method was employed with an objective of developing microparticles for oral controlled release and subjected for evaluation in terms of drug content, encapsulation efficiency, size analysis, compatibility studies and Invitro release studies (Behera,2008)
1) To prepare Glimepride loaded Eudragit RLPO microparticles by varying proportions of Polymer and surfactants.
2) To evaluate Glimepride loaded Eudragit RLPO microparticles (Sahoo 2005)
MATERIALS & METHODS:
1. The microparticles were prepared by emulsion solvent evaporation technique.
2. Glimepiride microparticleswere formulated by varying the drug and polymerratios and by varying the surfactants.
3. Weighed amount of drug and polymer were dissolved in 10ml of acetone.
4. The organic solution was then slowly added to 100ml of liquid paraffin containing 1% surfactant with constant stirring for 1hr.
5. The resulting microparticles were separated by filtration and washed with petroleum
ether.
6. The microparticles finally air dried over a period of 12 hrs and stored in a dessicator. (Davis 2004)

Characterization of Microparticles: (Dashora 2006)
Yield of Microparticles :
Microparticles recovered at the end of preparation were weighed and the yield was calculated as a percentage of the total amounts of polymer and drug added during the preparation of microparticles.
Flow properties: (Davis 2005)
Angle of Repose
Angle of repose is defined as the maximum angle possible between the surface of the pile of the powder and the horizontal plane. The flow characteristics of different microcapsules were studied by measuring the angle of repose employing fixed funnel method. The angle of repose was calculated by using the following formula. radius of the base of the pile
Tan θ = Height of the pile/ radius of base of the pile
where θ = tan-1 (h / r) θ = angle of repose.
Bulk Density & Tapped Density
Bulk density and tapped density were measured by using 10 ml of graduated cylinder. The pre weighed sample was placed in a cylinder; its initial volume was recorded (bulk volume) and subjected to tapings for 100 times. Then the final volume (tapped volume) was noted down. Bulk density and tapped density were calculated from the following formula.
Bulk density = Mass of microparticles / Bulk volume
Tapped density= Mass of microparticle / tapped volume
Carr’s Index
Compressibility index (CI) or Carr’s index value ofmicroparticles was computed according to the following equation: Carr’s index (%) = ((Tapped density – Bulk density) / Tapped density) * 100
Hausner’s Ratio (Pandey 2011)
Hausner ratio of microspheres was determined by comparing the tapped density to the bulk density using the equation:
Hausner's Ratio = Tapped density / Bulk density
Size Distribution and Size Analysis:
For size distribution analysis, 250 mg of the microparticles of different sizes in a batch were separated by sieving, using a range of standard sieves. The amounts retained on different sieves were weighed. The mean particle size of the microparticles was calculated by the formula.(Lin1999)
Estimation of drug content:( Hamaguchi 2004)
An accurately weighed portion of microparticles equivalent to 5 mg of Glimepiride were weighed and transferred in to a mortar. Powdered and dissolved in 100 ml of pH 7.4 phosphate buffer, suitably diluted the absorbance of the resulting solution was measured at 236 nm.
Entrapment Efficiency:
Entrapment efficiency was calculated using the formula;
Entrapment efficiency = (Estimated percent drug content / Theoretical Percent Drug Content) * 100 (Chowdary 2014)
Estimated percent drug content was determined from the analysis of microparticles and the theoretical percent drug content was calculated from the employedcore: coat ratio in the formulation of microparticles.(Nissen2008)
Morphological Characterization by SEM:
Morphology and surface characteristics were studied by Scanning Electron Microscopy. The samples for the SEM analysis were prepared by sprinkling the microparticles on one side of the double adhesive stub. The stub was then coated with fine gold dust. The microparticles were then observed with the scanning electron microscope (Leica Electron Optics, Cambridge, USA) at 10 kv). (Khan 2004)
Drug Release Studies:
Release of Glimepiride from the microparticles, was studied in phosphate buffer of pH 7.4 (900 ml) using Eight Station Dissolution Rate Test Apparatus (M/s. Electrolab) with a paddle stirrer at 100 rpm and at 37 OC ± 0.5 OC. A sample of microparticles equivalent to 5 mg of Glimepiride was used in each test. Samples were withdrawn through a filter (0.45) at different time intervals and were assayed at 228 nm for Glimepiride using Shimadzu double beam UV spectrophotometer. The drug release experiments were conducted in triplicate (Bravo2002).
Result Table 2: Micromeritic Properties of Glimepiride microparticles, Percentage yield, % drug content and % encapsulation efficiency of Glimepiride microparticles
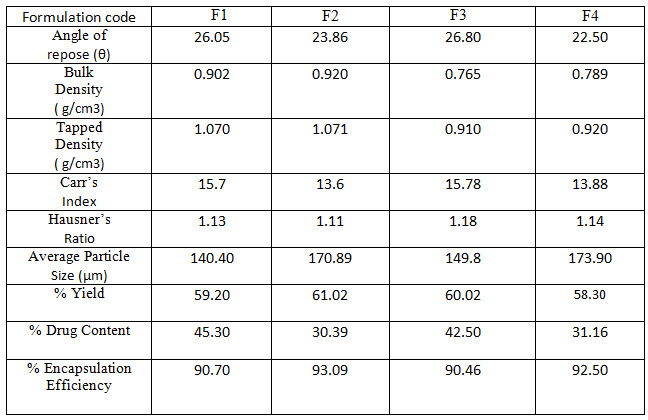
Table 3: Release Profiles of Glimepiride microparticulate (F1toF4)


NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
Glimepiride loaded Eudragit RLPO microparticles were successfully formulated by emulsion solvent evaporation method. In these formulations, span 80 and tween 80 are used as surfactants and the optimum concentration of each is 1% w/v. A total number of four batches were formulated by varying the process variables like change in polymer concentration and type of surfactant. The detailed composition of microparticles was shown in Table 1. These microparticles were evaluated for their percentage yield, flow properties, size analysis, percent drug content, percent encapsulation efficiency and morphological characterization, The angle of repose values of all the formulations were found to be in the range of 22.50 – 26,80, i.e. less than 30, which shows their free flowing nature of the prepared microparticles. Bulk density and tapped density showed good packability of the microparticles.
REFERENCE:
1. Behera B.C, Sahoo S. K, Dhal S, Barik B B, Gupta BK (2008) ; Characterization of Glipizide-Loaded Polymethacrylate Microspheres Prepared By an Emulsion Solvent Evaporation Method. Tropical Journal of Pharmaceutical Research ;7(1):879-885.
2. Bravo S.A, Lamas M.C, Salamon CJ. (2002) In-Vitro Studies of diclofenac Sodium controlledrelease from biopolymeric hydrophilic matrices. J Pharm apharm Sci ;5(3):213- 219.
3. Chowdary K.P, Rao YS. (2003); Design and in vitro and in vivo evaluation of mucoadhesive microcapsules of glipizide for oral controlled release: A technical note. AAPS PharmaSciTech ;4(3):E39.
4. Davis S.N. (2004) ; The role of glimepiride in the effective management of Type 2 diabetes. J Diabetes Complications ;18(6):367-376.
5. Davis, Stephen N. (2005), 60 ; Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas". In Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (eds.). Goodman & Gilman's the Pharmacological Basis of Therapeutics. New York: McGraw-Hill. 1636.
6. DashoraK, , Saraf S. (2006) ; Effect of processing variables on micro particulate system of aceclofenac. Pak J Pharm Sci ;19(1) : 6-10.
7. Hamaguchi T, , Tokunaga K et al., (2004) ; Efficacy of glimepiride in type 2 diabetic patients treated with glibenclamide. Diabetes Res Clin Pract ; 66(1):S129-132.
8. K.P.R Chowdary, K.Ravi Shankar, S.V.V. Subrahmanyam Recent research on Microparticles-A Review J.Pharma.Science Volume-5,Issue-2,pp-1557-1566-April-June(2014) . ShamSuddin
9. Lin WJ and Wu TL. (1999) ; Modification of the initial release of a highly water soluble drug from ethyl cellulose microspheres prepared by emulsion solvent evaporation method. Journal of Microencapsulation ;16:639-646
10. Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A et al., (2008) ; Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA ; 299(13):1561-1573.
11. Pandey A, Singh BV. (2011) ; Formulation development & optimization of Glimepiride microspheres using ionotropic gelation technique. Pharmacia ;1; 67-72.
12. Sai Kishore V, Gopala krishna murthy TE, Pavan Kumar A, Satyanaryana J. (2011) Formulation and evaluation of mucoadhesive microcapsules of glimepiride. Res J Pharm Tech ;4(5) :739 743.
13. Sahoo SK, Mallick AA, Barik BB, Senapati PC. (2005) ; Formulation and in vitro Evaluation of Eudragit Microspheres of Stavudine. Tropical Journal of Pharmaceutical Research ;4(1) :369 375.
14. Sultan Khan Md. Reserch Article J.advances in pharmaceutics (http://ijap.ssjournals.com)
15. Haznedar S, Dortunc B. (2004) ; Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm ; 269(1):131-140.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









