{ DOWNLOAD AS PDF }
ABOUT AUTHORS
P.Mounika*, Sanjit, Abhishek pandey
Gautham College of Pharmacy
Hebbal, Bengaluru, Karnataka
ABSTRACT:
All active pharmaceutical ingredients are having good therapeutic activity and show poor oral bioavailability , because of poor solubility .The present study is to investigate to improve the solubility of Felodipine using different carriers and different methods of preparation of techniques to identify that which carrier and suitable method of preparation. All formulation are evaluated for hardness, friability, drug content uniformity, and in vitro dissolution studies. Among all the formulations three formulation shows good drug release and the formulation with direct compression method shows good drug release compared to other formulation among all the formulation Polyvinylpyrrolidone (PVP) with direct compression is considered as ideal formulation from the study.
Reference Id: PHARMATUTOR-ART-2658
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 7, Issue 04 Received On: 08/03/2019; Accepted On: 14/03/2019; Published On: 01/04/2019 How to cite this article: Reddy, M., Yadav, S. and Pandey, A. 2019. Formulation and Evaluation of Felodipine tablets using different carriers and different methods of preparation for Enhancement of Solubility. PharmaTutor. 7, 4 (Apr. 2019), 42-47. DOI:https://doi.org/10.29161/PT.v7.i4.2019.42 |
INTRODUCTION
Felodipine is a dihydropyridine calcium antagonist with positive inotropic effects. It lowers blood pressure by reducing peripheral vascular resistance through a highly selective action on smooth muscle in arteriolar resistance vessels (Indian Pharmacopoeia, 4th Edn. Vol. II). Hypertension is a major risk for Cardiovascular and stroke complications. The good permeability and the poor water solubility (BCS class II) as well as short biological half-life. Felodipine is an orally administered drug and is almost completely absorbed and undergoes extensive first pass metabolism. The systemic bioavailability is approximately 20%. Mean peak concentration reaches in 2.5 - 5 hrs.
MATERIALS AND METHODS
Felodipine kind gift sample from Gautham College of pharmacy
Avicel PH 102, PVP, PEG 6000, Cross povidone, starch, lactose
From Gautham college of pharmacy.
METHOD OF PREPARATION OF FELODIPINE TABLETS
Tablets prepared by Dry granulation method
Granulation is a process in which powder particles are made to adhere to each other, resulting in larger, multi-particle entities, so called granules. If such a process is performed without adding liquids, this is called dry granulation. In dry granulation, the powder blend is compacted by applying a force onto the powder, which in general causes a considerable size enlargement. Principally there are two methods to obtain the compacts when using dry granulation: slugging and roller compaction (Valleri M. et al 2004; 30(5): 525- 534). Roller Compaction is used to prepare granules. A Roller compactor generally consist of three major units: A feeding system, which conveys the powder to the compaction area between the rolls .A compaction unit, where powder is compacted between two counter rotating rolls to a ribbon by applying a force .A size reduction unit, for milling the ribbons to the desired particle size. In this method starch, sodium starch Glycolate, Avicel PH 102 used as a carriers for Felodipine.
Tablets prepared by direct compression
In direct compression method, all ingredients as shown in Table 1 were mixed in geometrical order and compressed by using round flat punches on rotary tableting machine. In direct compression Avicel PH 102, Polyvinylpyrrolidone (PVP), Polyethylene glycol (PEG 6000), are used as a carrier for Felodipine tablets for enhancing the solubility of tablets (Chowdary KPR, Rao SS. 2002).
Tablets prepared by wet granulation
Avicel PH 102, Polyvinylpyrrolidone (PVP), Polyethylene glycol (PEG 6000), are used as a carrier for wet granulation. Starch solution used as binder for preparation (British Pharmacopoeia 1993). Granules are prepared by mixing drug, carrier, lactose and binder solution to get coherent mass ,granules are passed through the sieve number 44.talc and magnesium stearate starch powder are added in the final step.
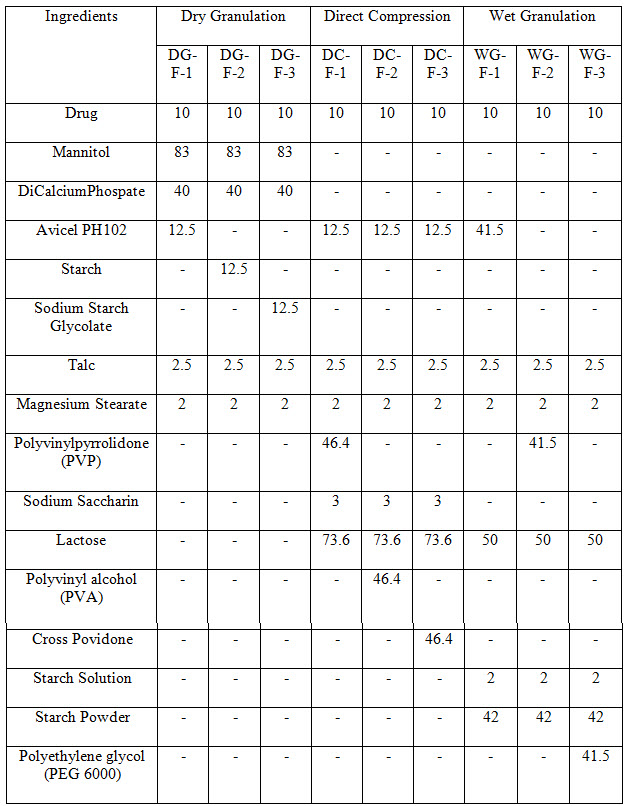
Table 1 Formulations of Felodipine
CALIBRATION CURVE OF FELODIPINE
Accurately weighed 10 mg of powdered drug was transferred to 100 mL volumetric flask. To this about 20 mL of ethanol was added and contents of the flask were shaken to effect the solution (United States Pharmacopoeia2004). Finally volume in the flask was made up to the mark by using ethanol (R. M. Silverstein, et al 1981).
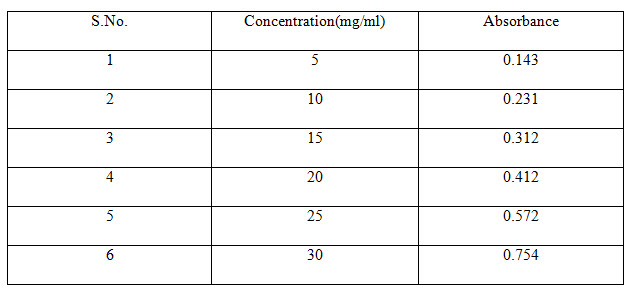
Table 2 Standard Absorbance of Felodipine
EVALUTION PARAMETERS
Tablet thickness and weight variation
Vernier caliper was used to determine tablet thickness. Tablet was placed in between two arms of the caliper. Average of three values was calculated (J. P. Blitz). Weight variation is determined by taking twenty tablets and weighing them on electronic weighing balance to determine the average weight. At the end, the individual weight was compared with average weight. (Seager H. J. Pharm. Pharmacol. 1998; 50: 375-382).
Friability
Friability of tablets was determined by using Roche Friabilator (Pharma Test, Germany). Twenty tablets were weighed and placed in the drum of the Friabilator and speed was adjusted at 25 rpm (Chang RK, et al Pharm Technol 2000; 24:52‐8.). The tablets were allowed to revolve, fall from height of six inches for 4 min. Then tablets were de-dusted using muslin cloth and re-weighed.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Tablet disintegration
Tablet disintegration apparatus was used. Six tablets were taken and placed individually in tubes and properly covered. The temperature of medium was maintained at 37±2° and timely noted by thermometer (Takao M, 1996). The time taken by the tablet to disintegrate completely was noted.
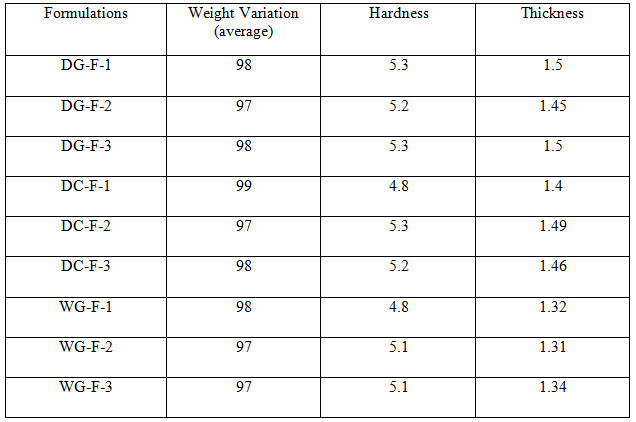
Table 3 Evaluation Parameters
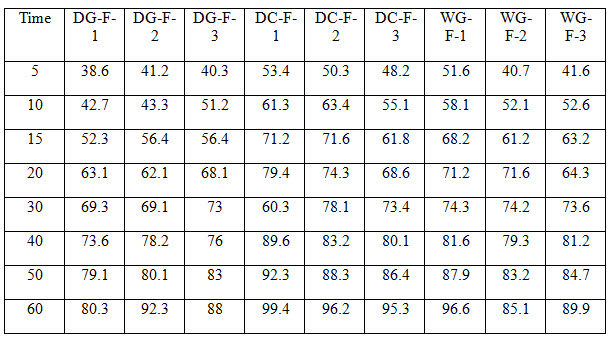
Table 4 Dissolution Profile for all Formulations
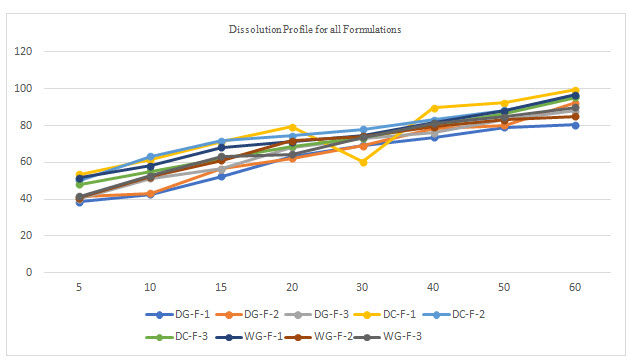

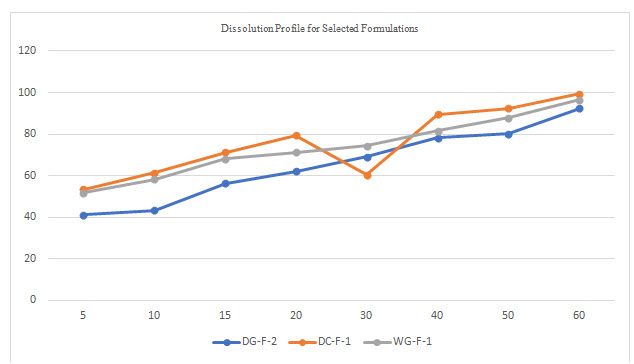
Table 5 Dissolution data for selected formulation
Dissolution study
Dissolution studies were performed on USP type-II apparatus. The speed of apparatus was set at 50 rpm.900ml of phosphate buffer solution of pH 6.8 was taken as dissolution medium in each vessel of the apparatus ( Zhao N, Augsburger LL. Et al. Article 79) Dissolution vessels and temperature of medium was kept at 37±0.5°. The dissolution sample was taken from each vessel at regular intervals and was replaced by equal quantity of freshly prepared media (Sunada H et al .2002). Absorbance was measured by using UV/Vis spectrophotometer.
RESULTS AND DISCUSSION
All the tablets of Felodipine were subjected to weight variation, friability, drug content uniformity, and in vitro dissolution .Tablets were prepared by three different methods direct compression ,dry granulation, and wet granulation ,using different carriers for enhancing the solubility of Felodipine. All tablets shows +- 1% weight variation, tablets prepared by different carriers shows sufficient hard ness, thickness 2% variation. In formulations with dry granulation Avicel, sodium starch Glycolate and starch use as carriers. Among these DG- F-2 shows percentage drug released is more compared to other formulations. Starch with Felodipine gives good dissolution data. In direct compression Felodipine with Avicel PH 102 shows good drug release i.e. DC – F -1.In wet granulation WG-F-1 gives more drug release in 60min. Again dissolution studies were carried out between these three formulations to get the average .from the data direct compression with PVP show good dissolution characteristics.
CONCLUSION
For enhancing the solubility of Felodipine with different carriers shows decrease in disintegration time and dissolution time. The aim of this study is to evaluate the solubility of Felodipine using different carriers and different methods. Felodipine is poorly soluble in water. For enhancing the solubility carriers like Polyvinylpyrrolidone, PolyethyleneGlycol 6000, Avicel PH 102, sodium starch Glycolate, starch, polyvinyl alcohol (PVA). Tablets prepared with dry granulation shows 92% dissolution in 60min, wet granulation 96.6% dissolution in 60 min, due to drawback associated with wet granulation is microbial contamination. All formulation direct compression with Avicel PH 102 is a suitable carrier for Felodipine tablets for enhancing the solubility.
REFERENCES
1. British Pharmacopoeia 1993 (BP1993), Appendix XVII, 13. An HPLC/FTIR interface is available commercially from Lab Connections, Inc., Marlborough, MA.
2. Chang RK, Guo X, Burnside B, Couch R. Fast dissolving tablets. Pharm Technol 2000; 24:52‐8.
3. Chowdary KPR, Rao SS.(2000); Investigation of dissolution enhancement of itraconazole by solid dispersion in superdisintegrants; Drug Dev Ind Pharm; 26(11); 1207‐11.
4. Indian Pharmacopoeia, 4th Edn., Controller of publications, India, New Delhi, 1996, A‐80.
5. Indian Pharmacopoeia, 4th Edn., Vol. II: Controller of Publications, Govt. of India, Ministry of Health & Family Welfare, New Delhi; 1996
6. J. P. Blitz and S. M. Augustine, Spectroscopy, 9, no. 8 (1994), 28.
7. R. M. Silverstein, G. C. Bassler, and T. C. Morrill, Spectrometric Identification of Organic Compounds, 4th ed. (New York: Wiley, 1981), 166.
8. Seager H. (1998); Drug delivery products and the zydis fast dissolving dosage forms; J. Pharm. Pharmacol. ; 50(4) ; 375-382.
9. Sunada H, Bi YX, Yonezawa Y, Danjo K. (2002); Preparation evaluation and optimization of rapidly disintegrating tablets; Powder Technol ; 122(2-3); 188‐98.
10. Takao M, Yoshinori M, Muneo F. Intra buccally dissolving compressed mouldings and production process thereof; US patent ; US5576014A
11. United States Pharmacopoeia. Rockville.MD:27th revision. USP Convention, Inc.; 2004. p. 2302.
12. Valleri M. Mura P. Maestrelli F. Cirri M. Ballerini R. (2004); Development and Evaluation of Glyburide Fast Dissolving Tablets using Solid dispersion Technique. Drug Dev Ind. Pharm; 30(5); 525- 534.
13. Zhao N, Augsburger LL. (2005); Functionality comparison of 3 classes of superdisintegrants in promoting aspirin tablet disintegration and dissolution; AAPS Pharm Sci Tech; 6(4); E634-40
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









