{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
U. A. Deokate, A. M. Gorde*
Dept. Of Pharmaceutical Chemistry,
Government College of Pharmacy, Hotel Vedant Road, Osmanpura,
Aurangabad, Maharashtra, India 431005
gordeanjali@gmail.com
ABSTRACT:
This review discusses the regulatory aspects of forced degradation and methodology aspects for degradant investigations. It also focuses on the prediction of degradation products and pathways and development of stability indicating assay method. While reviewing the analytical perspectives various conventional and hyphenated techniques for degradant separation and characterization are described in detail.
INTRODUCTION:
Chemical stability of pharmaceutical molecules is a matter of great concern as it affects the safety and efficacy of the drug product. Forced degradation studies provide data to support identification of possible degradants; degradation pathways and intrinsic stability of the drug molecule and validation of stability indicating analytical procedures. A draft guidance document suggests that results of one- time forced degradation studies should be included in Phase 3 INDs (Investigational New Drugs). NDA (New Drug Application) registration requires data of forced degradation studies as forced degradation products, degradation reaction kinetics, structure, mass balance, drug peak purity, etc. This forced degradation study provides information about degradation pathways of API, alone and in drug product, any possible polymorphic or enantiomeric substances and difference between drug related degradation and excipient interferences[1,2].
Controlling degradation related impurities involves identifying which of the potential degradation products found during forced degradation testing actually form in either the drug substance or product under long term or accelerated storage conditions and then selecting the appropriate counter measures to minimize the impurities or degradants. An impurity profiling study of forced degradation samples of drug substance illustrates the identification process and its potential impact on pharmaceutical development.
In the exercise of controlling impurities/degradants, their identification and characterization are the two key steps. These are required to be done when impurities/degradants are present at the prescribed stringent limits of 0.1%, or even lower for those genotoxic in nature. The conventional approach encompasses separation of impurities/degradants by a suitable method and their identification with the help of standard material. Alternatively, they are either enriched or isolated, followed by characterization through spectral analysis. The more modern concepts their characterization through the use of hyphenated tools. [3, 4]
FORCED DEGRADATION
Knowledge of the stability of molecule helps in selecting proper formulation and package as well as providing proper storage conditions and shelf life, which is essential for regulatory documentation. Forced degradation is a process that involves degradation of drug products and drug substances at conditions more severe than accelerated conditions and thus generates degradation products that can be studied to determine the stability of the molecule.[5]
1. Regulatory perspectives of forced degradation
A. From a regulatory perspective, forced degradation studies provide data to support the following:
• Identification of possible degradants
• Degradation pathways and intrinsic stability of the drug molecule
• Validation of stability indicating analytical procedures.
B. Issues addressed in regulatory guidances include:
• Forced degradation studies are typically carried out using one batch of material.
• Forced degradation conditions are more severe than accelerated stability testing such as
>50 °C; ≥75% relative humidity; in excess of ICH light conditions; high and low pH, oxidation, etc.
• Photostability should be an integral part of forced degradation study design.
• Degradation products that do not form in accelerated or long term stability may not have to be isolated or have their structure determined.
• Mass balance should be considered.
C. Issues not specifically addressed in regulatory guidance:
• Exact experimental conditions for forced degradation studies (temperatures, duration, and extent of degradation, etc.) are not specified.
• Experimental design is left to the applicant's discretion.[6, 7]
2. How much degradation is enough?
The question of how much stressing is enough has been the subject of much discussion amongst pharmaceutical scientists. In general, values anywhere between 5% to 20% degradation of the drug substance have been considered as reasonable and acceptable for validation of chromatographic assays.[8,9] However, for small pharmaceutical molecules for which acceptable stability limits of 90% of label claim is common, pharmaceutical scientists have agreed that approximately 10% degradation is optimal for use in analytical validation.[10] In the event that the experimental conditions generate little or no degradants due to the exceptional stability of the molecule, an evaluation should be made to verify if the drug substance has been exposed to energy in excess of the energy provided by accelerated storage (i.e., 40°C for 6 months). If the answer is yes, then the experiment can be stopped and a note of the stability of the drug substance can be made. Unduly overstressing the drug substance may produce aberrant results.[11]
3. Strategies for selection of forced degradation conditions [12]
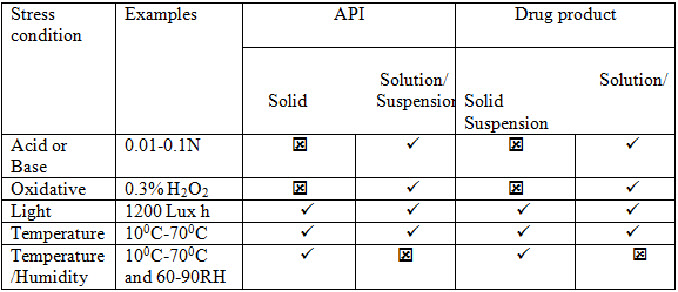
Table 1. Strategies of selection of forced degradation conditions.
A. Hydrolytic degradation:
Hydrolysis is a chemical process that includes decomposition of a chemical compound by reaction with water. Hydrolytic study under acidic and basic condition involves catalysis of ionizable functional groups present in the molecule. Acid or base stress testing involves forced degradation of a drug substance by exposure to acidic or basic conditions which generates primary degradants in desirable range. The selection of the type and concentrations of acid or base depends on the stability of the drug substance. Hydrochloric acid or sulfuric acids (0.1–1 M) for acid hydrolysis and sodium hydroxide or potassium hydroxide (0.1–1M) for base hydrolysis are suggested as suitable reagents for hydrolysis.[13, 14]
Hydrolysis of most of the drugs is dependent upon the relative concentration of hydronium and hydroxyl ions such as i) Anastrozole, significantly degraded in basic conditions as compared to acidic conditions and two new degradation products were formed under basic pH, ii)Doxofylline, a bronchodilator drug that show degradation more in acidic condition. Hence pH at which each drug is optimaly stable can be determined. [15, 16]
B. Photo degradation:
According to ICH Q1B guideline for photo degradation, samples should be exposed to light providing an overall illumination of not less than 1.2 million lux hours and an integrated near ultraviolet energy of not less than 200 watt hours/square meter with spectral distribution of 320-400nm to allow direct comparisons to be made between the drug substance and drug product. Samples may be exposed side-by-side with a validated chemical actinometric system to ensure the specified light exposure is obtained, or for the appropriate duration of time when conditions have been monitored using calibrated radiometers/lux meters.[17]
The photolytic degradation can occur through nonoxidative or oxidative photolytic reaction. The nonoxidative photolytic reactioninclude isomerization,dimerization, cyclization, rearrangements, decarboxylation and hemolytic cleavage of X-C hetero bonds, N-alkyl bond (dealkylation and deamination), SO2-C bonds etc and while oxidative photolytic reactionoccur through either singlet oxygen(1O2) or triplet oxygen(3O2) mechanism. The singlet oxygenreacts with the unsaturated bonds, such as alkenes, dienes, polynuclear aromatic hydrocarbon to form photoxidative degradation products whereas triplet oxygenreact with free radical of the drug molecule, which than react with a triplet oxygen molecule to form peroxide. Hence, light can also act as a catalyst to oxidation reactions.[18,19]
C. Oxidative degradation:
Many drug substances undergo autoxidation i.e., oxidation under normal storage condition and involving ground state elemental oxygen. Therefore it is an important degradation pathway of many drugs. Autoxidation is a free radical reaction that requires free radical initiator to begin the chain reaction. Hydrogen peroxide, metal ions, or trace level of impurities in a drug substance act as initiators for autoxidation. [20]
Selection of an oxidizing agent, its concentration, and conditions depends on the drug substance. It is reported that subjecting the solutions to 0.1–3% hydrogen peroxide at neutral pH and room temperature for seven days or upto a maximum 20%degradation could potentially generate relevant degradation products.[14]
The mechanism of oxidative degradation of drug substance involves an electron transfer mechanism to form reactive anions and cations. Amines, sulphides and phenols are susceptible to electron transfer oxidation to give N-oxides, hydroxylamine, sulphones and sulphoxide.[18]The functional group with labile hydrogen like benzylic, carbon, allylic carbon, and tertiary carbon or α – positions with respect to hetero atom is susceptible to oxidation to form hydroperoxides, hydroxide or ketone.[21,22]
D. Thermal degradation:
The thermal degradation studies carried out in dry or moist environment with temperature range of 40-700 C and moisture of 60-75%RH.[23] Thermal degradation study is carried out at 40°C to 80°C. The most widely accepted temperature is 70°C at low and high humidity for 1-2 months. High temperature (>80°C) may not produce predictive degradation pathway.[24] The use of high-temperatures in predictive degradation studies assumes that the drug molecule will follow the same pathway of decomposition at all temperatures.[19]
Effect of temperature on thermal degradation of a drug is studied through Arrhenius equation:
K= Ae-Ea/RT
Where k is specific reaction rate, A is frequency factor, Ea is energy of activation, R is gas constant (1.987 cal/deg mole) and T is absolute temperature. [20, 25]
· Microwave as new tool for thermal degradation study:
During the past ten years microwave-assisted chemistry has emerged as a very efficient and powerful technology to heat reaction mixtures in dedicated sealed reaction vessels/reactors. Theability to rapidly superheat solvents far above their boiling point up to 300 °C and 30 bar utilizing modern microwave instrumentation has been shown to dramatically reduce processing times compared to conventionally heated experiments under reflux conditions. Somewhat surprisingly, the exploitation of microwave technology for forced degradation/stress studies has scarcely been reported in the literature, with the only two published protocols describing the use of domestic microwave ovens without temperature control. Microtiter platform made out of strongly microwave absorbing silicon carbide (SiC) plates providing bore holes with the appropriate dimensions to be fitted with 20 standard HPLC/GC vials to perform low-volume microwave-assisted forced degradations (0.5–1.5 ml). The HPLC/GC vials are sealed with aluminum crimp caps equipped with PTFE coated silicone septa and an additional sealing mechanism enables parallel high-temperature processing of 80 vials up to 200 °C and 20 bar. [26]
4. Prediction of degradation pathways and product:
The process of prediction starts with the submission of a sample containing a degradation problem. A valuable first step of the process is an in-silico (computer software) and in-cerebro (chemistry knowledge) prediction of the potential reactive functional groups and degradation pathways of the drug molecule.
A. Predictive softwares (in-silico prediction)
1. CAMEO (Computer-Assisted Mechanistic Evaluation of Organic reactions):
Historical degradation predictions involved the use of for modeling and predicting organic chemical reactivities, software developed by William L. Jorgensen.[27-30]This software was discontinued, since predictions often overlooked secondary or ternary degradants, and its major downfall was the inability to program the software with new chemistry reactions.
2. DELPHI (Degradation Expert Leading to Pharmaceutical Insight):
It was another historical expert system, capable of predicting reaction products under given conditions. In contrast to CAMEO, DELPHI was specifically designed to predict reactivity and degradation of molecules[31]and proceeded beyond a primary reactive degradant to subsequent degradants of degradants. Even though described in the literature, DELPHI is a proprietary software system at Pfizer that has been discontinued due to its inflexibility.[32]
3. Zeneth:
This In-silico software released in 2010 is the only commercially available program designed to predict degradation pathways of pharmaceutical compounds. It was developed by Lhasa Ltd. in consortium with a group of pharmaceutical companies and based on the framework of Meteor, a metabolite-prediction software program by Lhasa.[32]Zeneth contains a chemical engine allowing the description and application of degradation transformations, a reasoning engine allowing the description and application of degradant transformation, a resoning engine allowing assessment of transformation likelihoods and graphical interface allowing entry of query structures and display of prediction results. Zeneth predicts degradation under the influence of reaction conditions and optionally in the presence of other compounds such as excipients.
Two of the main advantages of Zeneth are, total recall and the absence of bias. A further major benefit is the steady accumulation of knowledge about degradation chemistry in an accessible form. [33]
Wet or bench chemistry is still needed and the power of prediction can be harnessed to develop focused stress-testing protocols and to serve as a tool for the structure elucidation scientist to match predicted degradant structures with high performance LC-MS data.
B. In-cerebro prediction:
In-cerebro tools of great utility have been published in reviews and books in the primary literature.[34-37]In these references, the major mechanisms of chemical decomposition of pharmaceuticals have been examined in the context of common functional groups. The major mechanisms of chemical decomposition of pharmaceuticals include hydrolysis, dehydration, oxidation, isomerization/epimerization, decarboxylation, dimerization, polymerization, and photolysis and transformation products involving reaction with excipients/salt forms. While many pathways of degradation are obvious from basic organic chemistry principles, it is notuncommon to find surprising degradation chemistry leading to unexpected degradation products and pathways. Drug degradation prediction will improve as the field of degradation chemistry matures with documentation of investigational experiments in the literature. [32]
C. Drug degradation database:
The concept of a drug degradation database was developed at Pfizer and was initially designed to contain structure-elucidation data to allow scientists to retrieve data readily based on change of structure and degradation-chemistry conditions. This was accomplished using CambridgeSoft Corporation’s ChemOffice WebServer product, a comprehensive Windows-based program, which was capable of achieving the degradation database goals.[38]These include a process for the transfer of degradation results into one organized record per API and the ability to perform partial and full chemical-structure searches, and searches based on degradation conditions.
Pharmaceutical companies can now maintain a proprietary degradation database housed on their internal server or share degradation related information (i.e. chemistry from the literature and conferences) with other companies on a server maintained by CambridgeSoft [32]and referred to as Pharma D3. As the database grows, it is a useful tool for the field of degradation chemistry, enabling searches of specific drugs and molecular scaffolds, as well as uncovering patterns of degradation of specific functional groups and of drugs in general.[34]
In general we can describe the flow of forced degradation in the form of a map as follows,
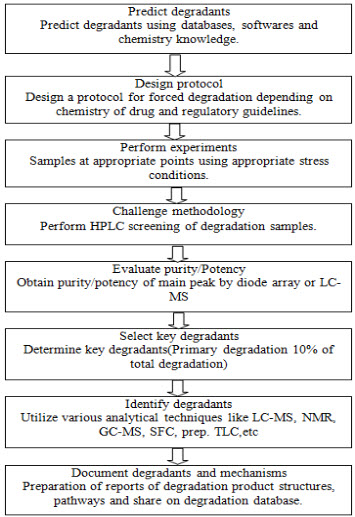
Fig.1. Forced degradation process flow map [7]
STABILITY INDICATING METHOD
The stability-indicating assay is a method that is employed for the analysis of stability samples in pharmaceutical industry. With the advent of International Conference on Harmonization (ICH) guidelines, the requirement of establishment of stability-indicating assay method (SIAM) has become more clearly mandated. [39]
A stability-indicating method is defined as an analytical method that accurately quantitates the active ingredients without interference from degradation products, process impurities, excipients, or other potential impurities. A method that accurately quantitates significant degradants may also be considered stability-indicating. A proactive approach to developing a stability indicating HPLC method should involve forced degradation at the early stages of development with the key degradation samples used in the method development process. [7]
Forced degradation should be the first step in method development. If forced degradation studies are performed early, method development and identification of primary degradation products and unknown impurities can be run in parallel. Using this process, a validated HPLC analytical assay, mechanisms of degradation, and the impurity/degradant information for filing can all be generated.
1. Development of stability indicating method
Though the requirements with respect to stability indicating method have been spelt out in regulatory documents, information on the basic steps to be followed for the development and validation of stability-indicating methods is neither provided in the regulatory guidelines nor in the pharmacopoeias. General steps in stability indicating method are,
Step1: Critical study of the drug structure to assess the likely decomposition route
This should be the first element whenever one takes up the project on establishment of a SIM. Much information can simply be gained from the structure, by study of the functional groups and other key components. There are definite functional group categories, like amides, esters, lactams, lactones, etc. that undergo hydrolysis [40], others like thiols, thioethers, etc. undergo oxidation, and compounds like olefins, aryl halo derivatives, aryl acetic acids, and those with aromatic nitro groups, N-oxides undergo photodecomposition.[41]
Step 2: Collection of information on physicochemical properties
Before method development is taken up, it is generally important to know various physicochemical parameters like pKa, log P, solubility, absorptivity and wavelength maximum of the drug in question.
Step 3: Stress (forceddecomposition) studies
As described above in forced degradation section, these studies should be carried out in accordance with ICH Q1A guideline. Stress conditions are (i) 10 °C increments above the accelerated temperatures (e.g. 50 °C, 60 °C, etc.), (ii) humidity where appropriate (e.g. 75% or greater), (iii) hydrolysis across a wide range of pH values, (iv) oxidation and (v) photolysis.[12]
Step 4: Preliminary separation studies on stressed samples
The simplest of separation way is to start with a reversed-phase octadecyl column and perform HPLC separation using UV/PDA detector system. Another way is to go for LC-MS separation.
Using these chromatographic techniques, one should follow the changes in all the stress samples at various time periods. The results should be critically compared with the blank solutions injected in a similar manner. It should be observed whether the fall in drug peak is quantitatively followed by a corresponding rise in the degradation product peaks.
Step 5: Final method development and optimization
Subsequent to preliminary chromatographic studies, the RT and relative retention times (RRT) of all products formed should be tabulated for each reaction condition. Special attention is then paid to those components whose RT or RRT is very close. PDA spectra or LC-MS profile of such components are obtained and critically evaluated to ascertain whether the products are same or different.
To separate close or co-eluting peaks, the method is optimized, by changing the mobile phase ratio, pH, gradient, flow rate, temperature, solvent type, and the column and its type. [39]
Step 6: Identification and characterization of degradation products, and preparation of standards
To identify the resolved products, a conventional way is to isolate them and determine the structure through spectral (MS, NMR, IR, etc.) and elemental analysis. However, this approach is tedious and time consuming when multiple degradation products are formed. Against it, the modern approach is to use hyphenated LC techniques coupled with mass spectrometry. This strategy integrates in a single instrument approach, analytical HPLC, UV detection, full scan mass spectrometry (LC-MS) and tandem mass spectrometry (LC-MS-MS) and provides a fair idea on identity of resolving components. These days a further integrated approach is becoming popular wherein LC-MS or LC-MS-MS is employed to obtain molecular weight and fragmentation information, and further detailed structural information is obtained through LC-NMR analysis.
Step 7: Validation
Validation of analytical methods, in general, has been extensively covered in the ICH guidelines Q2A and Q2B [42, 43], in the FDA guidance [44] and by USP [45].
The main focus of validation at this stage is on establishment of specificity/selectivity, followed by other parameters like accuracy, precision, linearity, range, robustness, etc. The limits of detection and quantitation are also determined for degradation products to help in establishment of the mass balance.
2. Specificand Selective stability-indicating assay methods
There is lack of clarity on the terms used for differentiating the methods that measure quantitatively the component of interest in the sample matrix without separation, and the ones where separation is done of the drug as well all other degradation products.
Thus ‘Specific stability-indicating assay method (Specific SIAM)’ can be defined as “a method that is able to measure unequivocally the drug(s) in the presence of all degradation products, excipients and additives, expected to be present in the formulation.
The ‘Selective stability-indicating assay method (Selective SIAM)’ on the other hand can be defined as “a method that is able to measure unequivocally the drug(s) and all degradation products in the presence of excipients and additives, expected to be present in the formulation.”[46, 47]
ANALYTICAL TOOLS FOR DEGRADANT SEPARATION AND IDENTIFICATION
A. Conventional Techniques:
1. Thin layer chromatography (TLC)
For many years, preparative thin layer chromatography (TLC) has proven to be quite useful. TLC is fast, easy and inexpensive, but it has limited throughput in the amount of material that can be recovered for structure analysis. It is typically limited in use for MS-proposed structures.
2. Solid phase extraction (SPE)
It is a fast way to enrich and to simplify a sample matrix prior to isolation. The ease of using SPE has also made it valuable in the post-isolation process as a means of de-salting and removing bulk amounts of water from collected semi-preparative chromatographic fractions.
3. Accelerated solvent extraction (ASE)
It is an effective way to use organic solvents to extract API and impurities from a solid matrix in a timely manner.[48–50]Limitations included possible degradation of extracted compounds as a result of the use of high temperatures and high pressures.
4. Low-pressure LC (LPLC)
Flash chromatography (FC) is one of the low pressure chromatography and is a conventional technique of choice for cases when NMR analysis is required to support degradant identification. FC is a relatively inexpensive technique that can process milligram-to-gram quantities of material in a timely manner, however, is sufficient for separations that require only moderate resolution
5. Supercritical fluid extraction (SFE)
Using carbon dioxide and countercurrent chromatography (CCC) have found applications in both natural product and pharmaceutical industry structure elucidation workflows. CCC is a high-resolution chromatographic choice without a solid stationary phase and uniquely applicable to unstable compounds. [51]
6. Mass Spectrometry (MS)
MS is an essential tool in all structure elucidation workflows. This technique provides high sensitivity, high dynamic range, richness of information, and the ability to couple to LC separation ability to couple to LC separations directly and provide structural information “on the fly”.
MS instrumentation has seen much advancement in the past two decades, which have increased availability of the high-resolution instrument. Some 10 years ago, a state-of-the-art time-of flight (TOF) instrument had a typical resolution of 10000–20000. Recently available TOF instruments from multiple vendors offer routine resolution in the 40000–60000 range without sacrificing sensitivity. [52]
An iontrap instrument with multiple-stage fragmentation has been the standard for structure elucidation. A typical workflow for an unknown identification is first to carry out the fragmentation experiment for the API and then to assign its fragments to understand the fragmentation of the molecule. The same fragmentation experiment can then be carried out for the impurity and fragments for both the API and the impurity can be compared.
7. Nuclear Magnetic Resonance (NMR)
NMR spectroscopy is an extremely powerful tool for the analysis of drug degradation products.[53,55,72]Combining the detailed structural information provided by NMR spectroscopy with a molecular formula and additional structural insight from MS fragmentation experiments can prove extremely valuable in the workflow for drug-degradation products. In order to perform NMR-based structure elucidation of drug-degradant products, it is common practice to isolate sufficient material (>1 mg) for NMR analysis. One- and two-dimensional NMR experiments are then used to piece together correlated fragments of the molecule, allowing the molecular structure to be determined. With improvements in NMR-probe technology, it has become possible to perform these experiments on microgram quantities of isolated sample. [15]
8. High Performance Liquid Chromatography (HPLC)
HPLC is routine technique for separation of degradants. The normal UV HPLC detectors these days allow for simultaneous measurement at multiple wavelengths, and some of them even give output of ratio plots at two wavelengths. This technique has also been promoted for peak purity testing during development of SIMs. [46]
B. Hyphenated Techniques
1. GC-MS
GC-MS was the first technique to be hyphenated and continues to be indispensible for the confirmation of organic volatile IMPs [57-60]and residual solvents [61-63]present in a sample. However, the properties of analytes essential for GC-MS, like volatility and thermal stability, are not known pre-hand for most organic impurities and degradants. Therefore, only sporadic literature reports exist on the use of this tool in the characterization of pharmaceutical relevant impurities.
2. LC-MS
LC-MS and its variants are most popular among all the hyphenated techniques for impurity characterization, as they carry potential to yield near unequivocal structural information even on their own. The range of advance instrument with LC-MS is as follows,
• LC-MS (Single Quad).
• LC-MS-MS (Triple Quad).
• LC-TOF.
• LC-MS-TOF (Q-TOF, Triple TOFTM).
• LC-MS-3DTRAP (MSn).
• LC-MS-2DTRAP (Q-TrapTM).
• LC-Hybrid Trap TOF Systems (LCMS-IT-TOF®).
• LC-OrbitrapTM.
• LC-FTICR (Fourier Transform Ion Cyclotron Resonance).
Three general utilities of LC-MS systems whichprovide primary input towards structure elucidation of degradation products are high resolution mass spectrometry (HRMS), multi-stage mass spectrometry (MSn) and hydrogen/deuterium exchange mass spectrometry (HDE-MS). [3]
3. Capillary Electrophoresis- Mass Spectrometry (CE-MS)
Capillary electrophoresis (CE) and capillary electrochromatography (CEC) represent important orthogonal techniques for the separation of Impurities and degradation products. CEC is a hybrid technique involving high efficiency of CE, and mobile and stationary phase selectivity of LC. Systems wherein CE and CEC are hyphenated with MS are gradually gaining significance for the characterization of degradants, though yet their use is in exploratory phase and restricted to the development of methods of separation, study of the utility of different CE modes and judgment of the benefit of different types of mass spectrometers for the purpose. [64-66]
4.Liquid Chromatography- Nuclear magnetic Resonance (LC-NMR)
The coupling of LC effluent to NMR was reported for the first time in 1978. [67]Since then, several instruments have been installed in industry and research laboratories. Modern LC-NMRsystems are associated with multiple technological advancements, like use of strong field magnets, microprobes and cryoprobe technology [68-71]to improve instrument sensitivity and resolution. Magnets with 500 MHz and above field strength are common as attachments to LC. The flow-through microprobes are available in different inner diameters to allow for handling of variable sample volumes from LC. Cryogenic cooling helps in the detection of submicrogram quantities since decreasing the temperature increases the response.
The advantages of using NMR in combination with HPLC in comparison toHPLC-MS coupling are (1) both HPLC and NMR are conducted in solution and notransfer from one phase to another is, as from the liquid to vapor phase inHPLC-MS; (2) NMR measurements are not limited by vaporization and hence bymolecular weight; (3) in many cases the structure information by NMR spectra ismore extensive, especially when the stereochemistry of the molecule is also considered. [56]
5. Liquid chromatography-Fourier Transfer Infrared (LC-FTIR)
Recording of IR spectra conventionally requires 1–5 mg of the sample, hence the same becomes difficult when components are present/generated in minute quantities and cannot be isolated. This is the reason behind the advent of LC-IR systems, which have been commercialized only recently.
Literature reports on the use of LC-IR in the characterization of degradants are only few. Somsen et al. [73]evaluated the usefulness of the LC-FTIR system as a stand-alone technique in impurityprofiling. In this study, a stability sample of Testosterone undecanoate stored at 60 °C and 75% RH for 5 months and a fresh sample were subjected to LC-FTIR investigation.Interpretation of IR spectra and its comparison with the drug spectrum revealed absence of characteristic bandsof conjugated C-3 carbonyl (1675 cm−1) and adjacent conjugatedC= C (1610 cm−1) in the degradation product, indicating saturation of double bondin the steroid skeleton.
CONCLUSION:
The review described above discuses the importance of forced degradation in drug development stage. Further it overview the soft tools for prediction of degradation product and pathways. A properly designed and executed forced degradation study would generate an appropriate sample for development of stability indicating method. The above discussion clearly shows a definite shift from the conventional way of structure elucidation of impurities and degradation products (involving isolation and spectral analysis) to the use of modern hyphenated techniques. Future directions in pharmaceutical degradant profiling will involve improvements in both the technique-oriented and the chemistry-guided approaches.
REFERENCES:
1.R.N. Rao, A.N. Raju and R. Narsimha: Isolation and characterization of process related impurities and degradation products of bicalutamide and development of RP-HPLC method for impurity profile study. Journal of Pharmaceutical and Biomedical Analysis 2008; 46:505–519.
2.Deepti Jain and Pawan Kumar Basniwal: Forced degradation and impurity profiling: Recent trends in analytical perspectives. Journal of Pharmaceutical and Biomedical Analysis 2013; 86:11–35.
3.Saranjit Singh, Tarun Handa, Mallikarjun Narayanam, Archana Sahu, Mahendra Junwal and Ravi P. Shah: A critical review on the use of modern sophisticated hyphenated tools in the characterization of impurities and degradation products. Journal of Pharmaceutical and Biomedical Analysis 2012; 69:148– 173
4.Abolghasem Jouyban and Hamed Parsa: Genotoxic Impurities in Pharmaceuticals. Chapter no. 17, Toxicity and Drug Testing.
5.Blessy M1, Ruchi D Patel and Prajesh N Prajapati, Y K Agrawal: Development of forced degradation and stability indicating studies of drugs: A review. Journal of Pharmaceutical Analysis 2013; 09:003
6.ICH harmonised tripartite guideline stability testing of new drug substances and products Q1A (R2).
7.Karen M. Alsante, Akemi Ando, Roland Brown, Janice Ensing, Todd D. Hatajik, Wei Kong and Yoshiko Tsuda: The role of degradant profiling in active pharmaceutical ingredients and drug products. Advanced Drug Delivery Reviews 2007; 59:29–37.
8.Szepesi G: Selection of High-performance liquid chromatographic methods in pharmaceutical analysis. Journal of Chromatography 1989; 464:265-278.
9.Carr GP and Wahlich JC: A practical approach to method validation in pharmaceutical analysis. Journal of Pharmaceutical and Biomedical Analysis 1990; 86:613-618.
10.Jenke DR: Chromatographic method validation: a review of common practices and procedures II. Journal of Liquid Chromatography 1996; 19:737-757.
11.George Ngwa: Forced degradation as an integral part of HPLC stability-indicating method development. Drug delivery technology June 2010; vol.10, no. 5.
12.Dr. Hildegard Brümmer: How to approach a forced degradation study. Issue no.31, Life science technical bulletin January 2011.
13.S.Singh and M.Bakshi: Guidance on conduct of stress tests to determine inherent stability of drugs. Pharmaceutical Technology 2000; 24:1–14.
14.K.M. Alsante, A.Ando and R.Brown: The role of degradant profiling in active pharmaceutical ingredients and drug products. Advanced Drug Delivery Reviews 2007; 59(1):29–37.
15.Cheekatla S, Ravichandrababu R, and Reddy BN: Determination and characterization of degradation products of Anastrozole by LC–MS/MS and NMR spectroscopy. Journal of Pharmaceutical and Biomedical Analysis 2011; 56: 962-968.
16.Gupta A, Yadav JS, Rawat S and Gandhi M: Method Development and Hydrolytic Degradation Study of Doxofylline by RP-HPLC and LC-MS/MS. Asian Journal of Pharmaceutical Analysis 2011; 1:14-18.
17.ICH Harmonised Tripartite Guideline stability testing: Photostability testing of new drug substances and products Q1B .
18.Baertschi SW and Alsante KM: Stress testing: The chemistry of the drug degradation, pp: 99, in; Pharmaceutical Stress Testing, Baertschi SW, editors, Taylor & Francis, New York. 2005.
19.Ranjit Singh and Zia ur Rehman: Current trends in forced degradation study for pharmaceutical product development. Journal of Pharmaceutical Education and Research June 2012; vol.3, issue no.1.
20.Qiu F Norwood DL: Identification of pharmaceutical impurities. Journal of Liquid Chromatography R T 2007; 30: 877-935.
21.Boccardi G: Oxidative susceptibility testing, pp: 220. In; pharmaceutical Stress Testing Predicting Drug Degradation; Baertschi SW, editors, Taylor and Francis, New York. 2005.
22.Alsante KM, Hatajik TD, Lohr LL, Santafianos D and Sharp TR: Solving impurity/degradation problems: case studies. pp: 380, In; Handbook of Isolation and Characterization of impurities in Pharmaceutical, Ahuja S, Alsante K, editors, Academics Press, New York. 2003.
23.ICH Harmonised Tripartite Guideline stability testing of new drug substances and products Q1A (R2).
24.Dorman DE, Lornez LJ, Occolowitz JL, Spangle LA, Collins, MW, Bashore FN and Baertschi SW: Isolation and structure elucidation of the major degaradtion products of cefaclor in the solid state. Journal of Pharmaceutical Science 1997; 86: 526-539
25.Trabelsi H, Hassen IE, Bouabdallah S, Bouzouita K and Safta F: Stability indicating LC method for determination of Pipamperone. Journal of Pharmaceutical and Biomedical Analysis 2005; 39: 914-919.
26.Bojana Prekodravac, Markus Damm and C. Oliver Kappe: Microwave-assisted forced degradation using high-throughput microtiter Platforms. Journal of Pharmaceutical and Biomedical Analysis 2011; 56:867– 873.
27.T.D. Salatin and W.L. Jorgensen: Computer-assisted mechanistic evaluation of organic reactions: Overview. Journal of Organic Chemistry 1980; 45:2043–2051.
28.W.L. Jorgensen, E.R. Laird, A.J. Gushurst, J.M. Fleischer, M. Jan, S.A. Gothe, H.E. Helson, G.D. Paderes and S. Sinclair: CAMEO: A program for the logical prediction of the products of organic reactions. Pure Applied Chemistry 1990; 62:1921–1932.
29.J.M. Fleischer, A.J. Gushurst and W.L. Jorgensen: Computer-assisted mechanistic evaluation of organic reactions. 26: Diastereoselective additions: Cram’s Rule. Journal of Organic Chemistry 1995; 60:490-498.
30.S.A. Gothe, H.E. Helson, I. Houdaverdis, I. Lagerstedt, S. Sinclair and W.L. Jorgensen: Computer-assisted mechanistic evaluation of organic reactions: 22. The generation and use of three-dimensional structures. Journal of Organic Chemistry 1993; 58:5081–5094.
31.D.L. Pole, H.Y. Ando and S.T. Murphy: Prediction of drug degradants using DELPHI: an expert system for focusing knowledge. Molecular Pharmaceutics 2007; 4:539–549.
32.Christopher Foti, Karen Alsante, Guilong Cheng, Todd Zelesky and Mark Zell: Tools and workflow for structure elucidation of drug degradation products. Trends in Analytical chemistry 2013 49:89–99.
33.Martin A. Ott, Rob L. Toy and William G. Button: An Approach toward the Prediction of Chemical Degradation Pathways. Lhasa Limited, 22-23 Blenheim Terrace, Woodhouse Lane, Leeds LS2 9HD, United Kingdom.
34.S.W. Baertschi, K.M. Alsante and D. Santafianos: Stress testing: The chemistry of drug degradation, in: S.W. Baertschi, K.M. Alsante, R.A. Reed (Eds.), Pharmaceutical Stress Testing: Predicting Drug Degradation, second ed., Informa Health Care, New York, 2011; pp. 49–141.
35.M. Li: Organic Chemistry of Drug Degradation, RCS Publishing, Cambridge, 2012.
36.K.C. Waterman, R.C. Adami, K.M. Alsante, J. Hong, M.S. Landis, F. Lombardo and C.J. Roberts: Stabilization of pharmaceuticals to oxidative degradation. Pharmaceutical Development and Technology 2002; 7:1–32.
37.H.H. Tonnesson: Photostability of Drugs and Drug Formulations. CRC Press, Boca Raton, 1996.
38.K.M. Alsante, T.D. Hatajik, L.L. Lohr and T.R. Sharp: Isolation and identification of process related impurities and degradation products from pharmaceutical drug candidates. Part I, American Pharmaceutical Review 2001; 4:70–78.
39.Monika Bakshi and Saranjit Singh, Development of validated stability-indicating assay methods-critical review. Journal of Pharmaceutical and Biomedical Analysis 2002; 28:1011–1040.
40.K.A. Connors, G.L. Amidon and V.J. Stella (Eds.): Chemical Stability of Pharmaceuticals. Wiley, New York, 1986.
41.N.H. Anderson (Ed.): Photostability Testing: Design and Interpretation of Tests on Drug Substances and Dosage Forms. Taylor and Francis, London, 1996.
42.ICH, Text on Validation of Analytical Procedures. International Conference on Harmonisation, IFPMA, Geneva, 1994.
43.ICH, Validation of Analytical Procedures: Methodology. International Conference on Harmonisation, IFPMA, Geneva, 1996.
44.FDA, Guidance for Industry: Analytical Procedures and Methods Validation (Draft guidance). Food and Drug Administration, Rockville, MD, 2000.
45.The United States Pharmacopeia, 24th Revision, Asian Edition, United States Pharmacopeial Convention, Inc., Rockville, MD, 2000, pp. 2149–2152.
46.M. Grover, M. Gulati and S. Singh: Journal of Chromatography B: Biomedical Sciences and Application 1998; 708:153–159.
47.M. Grover, M. Gulati, B. Singh, S. Singh: Pharmacy and Pharmacology Communication 2000; 6:355–363.
48.A. Blanchard, C. Lee, B. Nickerson, L. Lohr, A. Jensen, K.M. Alsante, T.R. Sharp, D.P. Santafianos, R. Morris and K.D. Snyder: Identification of low-level degradants in low dose tablets. Journal of Pharmaceutical and Biomedical Analysis 2004; 36:265–275.
49.B.E. Richter: The extraction of analytes from solid samples using accelerated solvent extraction. LCGC 1999; 17:522–528.
50.M.A. Mottaleb, S.D. Sarker: Accelarated solvent extraction for natural products isolation. in: S.D. Sarker, L. Nahar (Eds.), Methods in Molecular Biology, Humana Press, New York, edition 3, 2012, 75–87.
51.R. Hu and Y. Pan: Recent trends in counter-current chromatography. Trends in Analytical Chemistry 2012; 40:15–27.
52. Ranasinghe, R. Ramanathan, J. Ragu, D. Mohammed, J. Celia, W.G. Humphreys and T.V. Olah: Integrated quantitative and qualitative workflow for in vivo bioanalytical support in drug discovery using hybrid Q-TOF-MS. BioAnalysis 2012; 4:511–528.
53.K.M. Alsante, S.W. Baertschi, M. Coutant, B. Marquez, T.R. Sharp and T.C. Zelesky: Degradation and impurity analysis for pharmaceutical drug candidates. in: S. Ahuja and S. Scypinski (Eds.): Handbook of Modern Pharmaceutical Analysis. Academic Press, San Diego, edition 2, 2011, 59–169.
54.M. Malet-Martino and U. Holzgrabe: NMR techniques in biomedical and pharmaceutical analysis. Journal of Pharmaceutical and Biomedical Analysis 2011; 55:1–15.
55.M. Elyashberg, A. Williams and G. Martin: Computer – assisted structure verification and elucidation tools in NMR-based structural elucidation. Progress in Nuclear Magnetic Resonance Spectroscopy 2008 53:1–104.
56.Ernst Bayer, Klaus Albert, Michael Nieder and Edgar Grom: On-line coupling of high-performance liquid chromatography and nuclear magnetic resonance. Journal o f Chromatography1 1979; 86:497-507.
57.N.L. Bochkareva, I.N. Glazkov and I.A. Revelsky: Combination of supercritical fluid extraction and gas chromatography-mass spectrometry: determination of impurities extracted from tablet preparations of the benzodiazepine series. Journal of Analytical Chemistry 2006; 61:1082–1089.
58.D. Djozan, A. Jouyban and J. Norouzi: Ultrasonic assisted SPME coupled with GC and GC-MS using pencil lead as a fiber for monitoring the organic volatile impurities of ceftazidime, Journal of Chromatographic Sciences 2008; 46:680–685.
59.K. Fliszar, J.M. Wiggins, C.M. Pignoli, G.P. Martin and Z. Li: Analysis of organic volatile impurities in pharmaceutical excipients by static headspace capillary gas chromatography. Journal of Chromatography A 2004; 1027:83–91.
60.G.L. McClure: Improved determination of organic volatile impurities in pharmaceutical materials by (USP-467) using automated static headspace GC/MS, PDA. Journal of Pharmaceutical Sciences and Technology 1999; 53:129–136.
61.Y. Liu and C.Q. Hu: Establishment of a knowledge base for identification of residual solvents in pharmaceuticals. Analytica Chimica Acta 2006; 575:246–254.
62.J.L.P. Pavon, M. Del, N. Sanchez, C.G. Pinto, M.E.F. Laespada and B.M. Cordero: Use of mass spectrometry methods as a strategy for detection and determination of residual solvents in pharmaceutical products. Analytical Chemistry 2006; 78:4901–4908.
63.J.L.P. Pavon, M. Del, N. Sanchez, M.E.F. Laespada, C.G. Pinto and B.M. Cordero: Analysis of class 1 residual solvents in pharmaceuticals using headspace programmed temperature vaporization-fast gas chromatography-mass spectrometry. Journal of Chromatography A 2007; 1141:123–130.
64. M.J. Hilhorst, G.W. Somsen, G.J. de Jong: Choice of capillary electrophoresis systems for the impurity profiling of drugs. Journal of Pharmaceutical and Biomedical Analysis 1998; 16:1251–1260.
65.J. Cai, J. Henion: Capillary electrophoresis-mass spectrometry. Journal of Chromatography A 1995; 703:667–692.
66.P. Hommerson, A.M. Khan, T. Bristow, M.W. Harrison, G.J. de Jong and G.W. Somsen: Drug impurity profiling by capillary electrophoresis/mass spectrometry using various ionization techniques. Rapid Communication in Mass Spectrometry 2009; 23:2878–2884.
67.N. Watanabe and E. Niki: Direct-coupling of FT-NMR to high performance liquid chromatography. Proceedings of Japan Academy Series B Physical and Biological Sciences 1978; 54:194–199.
68.K.L. Colson: Ultracool NMR technology. Modern Drug Discovery 2003; 47–51
69.H. Kovacs, D. Moskau and M. Spraul, Cryogenically cooled probes-a leap in NMR technology. Progress in Nuclear Magnetic Resonance Spectroscopy 2005; 46:131–155.
70.A.G. Webb: Microcoil nuclear magnetic resonance spectroscopy. Journal of Pharmaceutical and Biomedical Analysis 2005; 38:892–903.
71.R. Mukhopadhyay: Liquid NMR probes: Oh so many choices. Analytical Chemistry 2007; 79:7959–7963.
72.M. Malet-Martino and U. Holzgrabe: NMR techniques in biomedical and pharmaceutical analysis. Journal of Pharmaceutical and Biomedical Analysis 2011; 55:1–15.
73. G.W. Somsen, C. Gooijer, U.A.T. Brinkman, N.H. Velthorst and T. Visser: Coupling of LC and FT-IR: impurity profiling of testosterone undecanoate. Applied Spectroscopy 1992; 46:1514–1519.
REFERENCE ID: PHARMATUTOR-ART-2182
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 6 Received On: 07/04/2014; Accepted On: 15/04/2014; Published On: 01/06/2014 How to cite this article: UA Deokate, AM Gorde; Forced degradation and Stability Testing: Strategies and Analytical Perspectives; PharmaTutor; 2014; 2(6); 61-74 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









