{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Lagu surendra babu*, Prof. Y. Rajendra Prasad, D. Geetha mounika
Department of Pharmaceutical chemistry,
AU college of Pharmaceutical Sciences,
Andhra University, Visakhapatnam, AP, India
*ysbabu033@gmail.com
ABSTRACT:
Investigation of phytochemical and biological activities was performed by Antioxidant (DPPH, H2O2, Nitric oxide) free radical scavenging methods against three gradient polarity solvents extracts of leaves of Melia azaradach Linn with different concentraions. MA species of three different assay (DPPH, H2O2, Nitric oxide) of anti-oxidant activity were showed to be IC50 values of 20.14, 35.98, 36.43 with methanolic extract of leaves were have higher concentration possess more antioxidant potential than MALEoC IC50 42.85, 54.18, 30.56 and MALEoH IC50 values of 44.68, 52.71, 48.05 when compare to reference standard ascorbic acid IC50 values of 17.87, 18.91, 25.83. They exhibited strong antioxidant DPPH, H2O2, Nitric oxide radical scavenging activities with IC50 value reference ascorbic acid, MALoME, MALoCE and MALoHE respectively. Phytoconstituents like carbohydrates, flavonoids, proteins, saponins and tannins etc. An antioxidant activity of methanol extract could be due to the presence of flavonoids and phenols The results suggest that methanol and chloroform extracts from leaf melia azedarach tree have strong antioxidant potential.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2572
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 3 Received On: 24/01/2018; Accepted On: 25/01/2018; Published On: 01/03/2018 How to cite this article: Lagu SB, Prasad YR, Mounika DG; Evaluation of In Vitro Anti-Oxidant activity and Phytochemical Studies carried out for Melia Azedarach Leaf extracts; PharmaTutor; 2018; 6(3); 38-44; http://dx.doi.org/10.29161/PT.v6.i3.2018.38 |
INTRODUCTION:
An investigation of reactive oxygen species (ROS), play a critical role in the development of oxidative stress that can cause to many disorders including cardiovascular diseases, diabetes, inflammation, degenerative diseases, cancer, anemia, and ischemia etc (.Cai Y, et al., 2004). A free radical is a compound with more than one unpaired electrons in its outer most orbital. These unpaired free electrons species are unstable, suddenly reactive with other molecules due to the presence of unpaired electron species (Karlsson J, 1997). Therefore, they try to pair their electron(s) and generate a more stable compound.
The most harmful free radicals include atomic and molecular varieties of oxygen, known as Reactive Oxygen Species (ROS). ROS are emerged continuously in the body by both endogenous and exogenous factors like aerobic respirations, macrophages, polymorphic nuclear leukocytes and exposure to environment pollutants like organic solvents, pesticides, ionizing agent, tobacco smoke (Yeera R, et al., 2005) and (Beris H, 1991). Now a days ROS related to lipid peroxidation has been considered as one of the main causes of these diseases (Widodo N, et al., 2010).To prevent the cells against oxidative damage by free radicals, an antioxidant system, including superoxide dismutase (SOD), catalase, glutathione peroxidase, and glutathione reductase enzymes has evolved in aerobic organisms (Dickinson DA, 1991) and (Kurata M, et al. p. 1993).Under conditions of elevated ROS production or when the antioxidant system is compromised, cells are unable to scavenge the free radicals efficiently, leading to ROS accumulation. It has been demonstrated that many naturally occurring compounds possess notable activity as radical scavengers and lipid peroxidation inhibitors (Wagner, 1994). In addition to plant extracts, numerous naturally occurring compounds are useful as antioxidants, ranging from a vitamin A and E to plant antioxidants such as phenolic compounds, alkaloids, and organic sulfur compounds (SA, et al., 2006).
A large number of experiments have been carried out concerning the antioxidant activity of several plant extracts. The results of these experiments reveal that the activity is due to several secondary metabolites especially phenolic and flavonoids compounds. Melia azedarach commonly known as turaka vepa, belongs to the family of Meliaceae is widely distributed in Persia, India sub-continent and China, that is now naturalized in a number of continents including Africa, south-eastern Asia and large parts of northern and eastern Australia (Deepika Sharma, 2013) and (Porcher, et al.). The plant is traditionally used for the treatment of leprosy, inflammations, and cardiac disorders. Its fruits extracts possess ovicidal (Corpinella MC, 2007) and larvicidal activity (Wandscheer CB, 2004). The leaf extracts also possess antiviral (Descalzo AM, 1989). and antifertility activity (D.N, et al., 1990).To date, however, there has been little rigorous scientific study of this traditional medicine and indigenous plant and there is no scientific information on antioxidant properties of this leaves on different extracts of this plant. Thus, we evaluated the abilities of leaf extracts of Melia azedarach L. with different polarity solvents to function as an antioxidant agent by using vitro tests.
wwwwwwwww
MATERIALS AND METHODS
The leaves of Melia azedarach.L were collected from the kancharapalem gov.t poly technical college opposite to high way road beside area, Vishakhapatnam district, Andhra Pradesh. The plant was identified and authenticated with a code 22225 on 19-10-2016 by Dr. S.B. padal msc., PhD. Director of horticulture and gardening unit, Associate professor Botany Department, Andhra University herbarium(AUH), Vishakhapatnam district, Andhra Pradesh, India.
Preparation of crude drug for extraction
An authenticated of fresh leaves and tigs samples of plant M. azedarach L was collected at kancharapalem gov.t poly technical college opposite to high way road surrounding area, Vishakhapatnam district, Andhra Pradesh and washed individually under running tap water to remove any traces of soil particles and other dirt. The leaf was dried under shade at room temperature. Then macerated to coarse powder with a mechanical grinder and used to extract preparation. Passed through sieve. The powder was stored in an airtight container for further use.
Method of extraction
Macerate 1kg coarse powder leaves and for 48 hours with solvents (n-Hexane, chloroform and methanol) then reflux it for 1 hours at low temperature after that crude concentrate menstrum was separate it. Then filter, obtained menstrum subjected to rota vapour to get thick paste and recover the solvent again used to imbibition of coarse powder leaves. This procedure is carried for thrice. After that coarse powder leaves allowed to dry it successfully and then process same for remaining two solvents to macerate.
Pytochemical Analysis
Extract obtained using different solvent were subjected to the phytochemical screening of constituents by standard methods. Carbohydrates were identified by Molisch’s test; proteins were identified by Biuret test. Steroids, flavanoids, alkaloids, tannins, glycosides, sponins and fixed oils were identified by Libermann, Burchard test, Draggandroff’s test, Braymerr’s test, Lavau’s test, Haemolysis test, lead acetate test respectively
Chemicals and reagents
Gallic acid, ascorbic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), potassium ferricyanide, and FolineCiocalteu’s phenol reagent were obtained from SigmaeAldrich. Trichloroacetic acid, ferric chloride, ethanol, nhexane, chloroform, and ethyl acetate were purchased from Desai chemicals. Ferrous ammonium sulfate, AlCl3, sodium phosphate, ammonium molybdate, quercetin, and EDTA were purchased from SigmaeAldrich
In vitro antioxidant assay
In this study, several techniques have been used to determine the in vitro antioxidant activity to allow rapid screening of substances.
DPPH radical scavenging assay
The ability of three different MALoHE, MALoCE and MALoME to scavenge DPPH radicals was measured according to the method (Maxwell Afari Gyamfi, 1998) as previously described (Francis M., 2010). A solution of 0.3% DPPH was prepared in methanol and extract/fractions with different concentrations were mixed with 3 mL of DPPH solution. The changes in absorbance were measured after 30 minutes at 517 nm and the half maximal inhibitory concentration (IC50) value was calculated from the percentage inhibition values was calculate by comparing the absorbance values of the control and test samples using following equation:
S% = [(Acontrol – Asample) /Acontrol]×100
Where,
Acontrol = absorbance of the blank control (containing all reagents except the extract solution) Asample =absorbance of the test sample
Nitric oxide scavenging activity assay
Nitric oxide scavenging activity can be estimated by the use of Griess IlIosvoy reaction (rozina parual, 2016).The compound sodium nitroprusside is known to decompose in aqueous solution at physiological pH 7.2 producing NO¬. Under aerobic conditions, NO¬ reacts with oxygen to produce stable products (nitrate and nitrite). The quantities of which can be determined using Griess reagent. Scavengers of nitric oxide compete with oxygen leading to reduced production of nitrite ions. For the experiment, sodium nitroprusside (10mM) in phosphate buffered saline was mixed with different concentrations (10, 20, 30, 40, 50µg/ml) of MAHLE, MACLE, MAMLE of each plant were dissolved in methanol and incubated at 30ºC for 2 hours. The same reaction mixture without the extracts served as the control. After the incubation period, 0.5 ml of Griess reagent (1% sulfanilamide, 2% H3P04 and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride) was added. The absorbance of the chromophore that formed during diazotization of the nitrite with sulfanilamide and subsequent coupling with Naphthylethylenediamine dihydrochloride was immediately read at 550nm. Inhibition of nitrite formation by the gradient polar solvent extracts of the same plant and the standard antioxidant ascorbic acid were calculated relative to the control (Stanley mukangayama, 2014).
Hydrogen peroxide scavenging assay:
Principle:
Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups. Hydrogen peroxide can cross cell membranes rapidly, once inside the cell, H2O2 Can probably react with Fe2+, and possibly Cu2+ ions to form hydroxyl radical and this may be the origin of many of its toxic effects. It is therefore biologically advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate (Nagulendran, et al., 2017).
Method:
Scavenging activity of Hydrogen peroxide (H2O2) by the plant extract was determined by the method. Plant of different solvent extract (4 ml) prepared in distilled water at various concentration was mixed with 0.6 ml of 4 mM H2O2 solution prepared in phosphate buffer (0.1 M pH 7.4) and incubated for 10 min. The absorbance of the solution was taken at 230 nm. Ascorbic acid was used as a positive control compound. The percentage of inhibition was calculated by comparing the absorbance values of the control and test samples by above equation (Ruch R.J, 1989).
Table: 1 Results of Qualitative Phytochemical Screening of chloroform , hexane and Methanolic extracts of the leaves of Melia azedarach
|
Sn. No. |
Constituents |
Hexane Extract |
Chloroform Extract |
Methanol Extract |
|
01 |
Alkaloids |
+ |
+ |
+ |
|
02 |
Sterols |
+
|
+
|
+ |
|
03 |
Glycosides |
+
|
+
|
+
|
|
04 |
Fixed oil and fats |
+ |
+ |
+ |
|
05 |
Phenolic compounds |
+ |
+ |
+ |
|
06 |
Protein & amino acids |
+ |
+ |
+ |
|
07 |
Tannins |
+ |
+ |
+ |
|
08 |
Gum & mucilage |
+ |
+ |
+ |
|
09 |
Flavonoids |
+ |
+ |
+ |
|
10 |
Carbohydrates |
+ |
- |
+ |
|
11 |
Reducing sugars |
- |
+ |
+ |
|
12 |
Saponins |
- |
+ |
+ |
|
13 |
Tetraterpinoids |
+ |
- |
+ |
+ present, - absent
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Results of Antioxidant activity
DPPH free radical scavenging activity
The DPPH radical scavenging activity of MALoHE, MALoCE and MALoME was evaluate and compared with Ascorbic acid and the results are given in Table 2.1 The % inhibition at various concentration (10-50µg/ml) of MALoHE, MALoCE and MALoME as well as standard Ascorbic acid (10-50 µg/ml) were calculated and plotted in Figure 1 using Microsoft Office Excel 2010. The IC50 values are calculated from graph and were found to be 17.87 (standard Ascorbic acid) and 44.68, 42.85, 20.14 (MALoHE, MALoCE and MALoME) with respectively
Table 2.1: Results of DPPH scavenging activity
|
S.No. |
Concentraction μg/ml |
% Inhibition (MEAN±SEM) Of Melia azedarach leaf extracts |
|||
|
Hexane |
Chlorofrom |
methanol |
Std. ascorbic |
||
|
1 |
10μg/ml |
32.65±0.007 |
37.2±0.3 |
31.66±0.05 |
40.95±0.01 |
|
2 |
20μg/ml |
34.55±0.01 |
38.72±0.004 |
66.77±0.01 |
53.59±0.16 |
|
3 |
30μg/ml |
41.85±0.017 |
42.96±0.02 |
56.96±0.01 |
61.62±0.01 |
|
4 |
40μg/ml |
46.65±0.007 |
51.11±0.05 |
58.41±0.08 |
71.77±0.03 |
|
5 |
50μg/ml |
53.38±0.02 |
52.74±0.07 |
62.64±0.23 |
76.071±0.9 |
|
|
IC50 |
44.68 |
42.85 |
20.14 |
17.87 |
Fig 1: Results of DPPH scavenging activity

Results of Nitric Oxide scavenging activity
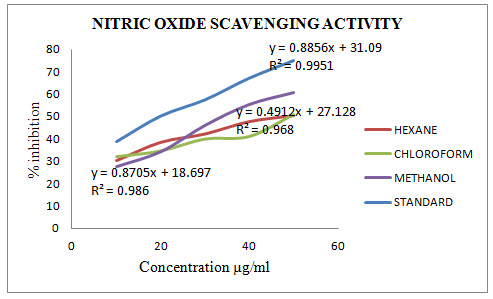
The nitric oxide scavenging activity of MALoHE, MALoCE and MALoME was evaluate and compared with Ascorbic acid and the results are given in Table 2.2. The percentage inhibition (% inhibition) at various concentration(10-50 µg/ml) of MALoHE, MALoCE and MALoME with the standard Ascorbic acid (10-50 µg/ml) were calculated and plotted in Figure 2 using Microsoft Office Excel 2010. The IC50 values are calculated from graph and were found to be 18.91 (Ascorbic acid) and52.71, 54.18, 35.98 (MALoHE, MALoCE and MALoME) with respectively.
Table 2.2: Results of nitric oxides scavenging activity
|
s.No. |
Concentraction μg/ml |
% Inhibition (MEAN±SEM) Of Melia azedarach leaf extracts |
|||
|
Hexane |
Chlorofrom |
methanol |
Std. ascorbic |
||
|
1 |
10μg/ml |
30.41±0.007 |
32.2±0.09 |
27.66±0.01 |
38.87±0.14 |
|
2 |
20μg/ml |
39.1±1.86 |
34.72±0.01 |
34.31±0.25 |
50.23±0.17 |
|
3 |
30μg/ml |
42.88±0.06 |
39.96±0.02 |
46.06±0.027 |
57.42±2.41 |
|
4 |
40μg/ml |
48.35±0.02 |
41.11±0.10 |
55.38±0.77 |
67.01±2.61 |
|
5 |
50μg/ml |
50.01±0.04 |
50.74±0.01 |
60.65±0.28 |
74.76±2.69 |
|
|
IC50 |
52.71 |
54.18 |
35.98 |
18.91 |
Fig 2: Results of nitric oxides scavenging activity
Hydrogen peroxide scavenging activity
The hydrogen peroxide scavenging activity of MALoHE, MALoCE and MALoME was evaluate and compared with Ascorbic acid and the results are given in Table 4. The percentage inhibition (% inhibition) at various concentration (200-500µg/ml) of three different MALoHE, MALoCE and MALoME as well as standard Ascorbic acid (12.5-100µg/ml) were calculated and plotted in Figure 3 using Microsoft Office Excel 2010. The IC50 values are calculated from graph and were found to be 25.83 (Ascorbic acid) and 48.5, 30.56, 36.43 (MALoHE, MALoCE and MALoME) with repectively.
Table 2.3: Results of hydrogen peroxides scavenging activity
|
S.No. |
Concentraction μg/ml |
% Inhibition (MEAN±SEM) Of Melia azedarach leaf extracts |
|||
|
Hexane |
Chlorofrom |
methanol |
Std. ascorbic |
||
|
1 |
10μg/ml |
26.41±0.15 |
27.62±0.02 |
25.09±0.03 |
32.25±0.12 |
|
2 |
20μg/ml |
36.22±0.32 |
30.72±0.05 |
33.28±0.01 |
47.01±0.02 |
|
3 |
30μg/ml |
40.38±0.18 |
42.96±0.51 |
41.52±0.02 |
53.69±0.02 |
|
4 |
40μg/ml |
47.1±0.07 |
51.11±0.41 |
57.87±0.31 |
66.98±0.68 |
|
5 |
50μg/ml |
49.6±0.14 |
53.74±0.14 |
61.41±0.09 |
71.66±0.25 |
|
|
IC50 |
48.05 |
30.56 |
36.43 |
25.83 |
Fig 3: Results of hydrogen peroxides scavenging activity

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
DISCUSSION AND CONCLUSION
Polyphenolic compounds are present in three different MALoHE, MALoCE and MALoME. It is well known that flavonoids and polyphenols are natural antioxidants but have also been reported to significantly increase SOD, glutathione and catalase activities. Furthermore it was shown that these compounds act as promoters for SOD, catalase and glutathione and cause the expression of SOD, glutathione.
From the Investigation it can be concluded that the gradient polar solvents like hexane, chloroform, methanol extracts of Melia azedarach (L.) possess antioxidant activity. So, the extracts was planned to be evaluated for in-vitro antioxidant activity. The in-vitro antioxidant potential of the three different solvents extracts of Melia azedarach L was evaluate by DPPH free radical scavenging activity, NO scavenging activity, H2O2 scavenging activity. Moreover the Ferric reducing power assay was also carried out which indirectly suggest the anti-oxidant potential of the concerned three different solvents extracts. The studies were carried out taking ascorbic acid as the standard antioxidant which is also a natural antioxidant. The results of antioxidant activity by DPPH free radical scavenging activity, NO scavenging activity, H2O2 scavenging activity were expressed in terms of % inhibition of generated free radicals respectively with respect to various concentrations. Concentration dependent effects were observed in each case i.e. higher concentrations were found to exhibit higher % inhibition in each protocol of the antioxidant study. The graphs were constructed with the % inhibition on X-axis verse concentrations on Y-axis. The IC50 value (50% inhibition) of the three MALoHE, MALoCE, MALoME and standard ascorbic acid were determined in all the studies. With standard ascorbic acid observed IC50 value of MALoHE, MALoCE and MALoME, the antioxidant potential was found to be linearly rise in following order-
H2O2 Scavenging Activity (IC50 - 48.5, 30.56, 36.43) > Nitric Oxide Scavenging Activity (IC50- 52.71, 54.18, 35.98) > DPPH Free Radical Scavenging Activity (IC50 - 44.68, 42.85, 20.14)
The scavenger assay of the MALoHE, MALoCE and MALoME suggested that it has the potential to reduce the ferric form. The absorbance value was found to be increased with increase in concentration of the extract and potent activity for methanol extract and chloroform extract.The further study on this plant might provide the isolation of some active constituents enduring the antioxidant potential.
REFERENCE
1. Beris H. “Antioxidant effects, a basis of drug selection.” Drugs, 42,1991,569
2. Cai Y, Luo Q, Sun M, Corke H; Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004,74:2157e2184.
3. Choudhary DN, Singh JN, Verma SK, Singh BP; Antifertility effects of leaf extracts of some plants in male rats. Indian J Exp Biol,1990, 28: 714.
4. Corpinella MC , Miranda M, Almiron WR, Ferrayoli CG, Almedia FL, Palacios SM; In vitro pediculicidal and ovicidal activity of an extract and oil from fruit of melia azedarach L. J Am Acad Dermatol 2007; 56: 250-6.
5. Deepika Sharma , Yash Paul; Preliminary and Pharmacological Profile of Melia azedarach L.: An Overview. Journal of Applied Pharmaceutical Science, 2013 Vol. 3 (12), pp. 133-138.
6. Descalzo AM, Coto C; Inhibition of the pseudorabies virus (scis herpesvinyl) by an vital agent isolated from the leaves of melia azedarach Rev. Argent Microbial 1989; 21: 133-40.
7. Dickinson DA, Forman HJ; Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488e504.
8. Fadzai Boora, Elaine Chirisa, Stanley; Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents, 2014.
9. Francis M. Awahl, Andrew W, Verla; Antioxidant activity, nitric oxide scavenging activity and phenolic contents of Ocimum gratissimum leaf extract, Journal of Medicinal Plants Research, 2010, Vol. 4(24), pp. 2479-2487, ISSN 1996-0875.
10. Karlsson J; “Introduction to nutralogy Radical Formation.” In: Antioxidants and Exercise .Illinois: Human Kinetics Press, 1997, 1-143.
11. Kurata M, Suzuki M, Agar NS; Antioxidant systems and erythrocyte lifespan in mammals. Comp Biochem Physiol B. 1993;106:477e487.
12. Maxwell Afari Gyamfi, Masato Yonamine, Yoko Aniya; Free-radical scavenging action of medicinal herbs from Ghana Thonningia sanguinea on experimentally-induced liver injuries, General Pharmacology, 32 (1999) 661–667.
13. Mohamed SA, Marzouk FA, Moharram MA, Mohamed AM, Gamal- Eldeen EA; Anticancer and antioxidant tannins from Pimenta dioica leaves, Natur Forsch, 2006;62:526e536
14. Nagulendran K, Velavan S, Mahesh R, Begum VH; In Vitro Antioxidant Activity and Total Polyphenolic Content of Cyperus Rotundus Rhizomes, 2007; 4(3): 440-449.
15. Porcher, Michel H (2012); "Melia names". Multilingual Multiscript Plant Name Database. University of Melbourne
16. Rozina Parul, Sukalayan Kumar Kundu, Pijush Saha; In Vitro Nitric Oxide Scavenging Activity Of Methanol Extracts Of Three Bangladeshi Medicinal Plants, the pharma innovate, 2013, Vol. 1 No. 12, pp.: 83, ISSN: 2277- 7695.
17. Ruch RJ, Cheng SJ, Klaunig JF; Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea, Carcinogenesis, 1989; 10: 1003–1008.
18. Wagner H, Fransworth NR. Economic and Medicinal Plant Research. London: Academic Press Limited; 1994;82:92e93.
19. Wandscheer CB, Duque JE, da silva MA; Larvicidal action of ethanolic extracts from fruits endocarps of melia azedarach and Azadirachta indica against the dengue mosquito Aedes Aegypti. Toxicol 2004; 44: 829-35.
20. Widodo N, Priyandoko D, Shah N, Wadhwa R, Kaul SC; Selective killing of cancer cells by Ashwagandha leaf extract and its componentWithanone involves ROS signaling. PLoS One. 2010, 5, e13536.
21. Yeera R, Senthil GP, Gupta M, Mazumdar UK. “Studies on in vitroantioxidant activities of methanol extract of Mucuna pruriens (Fabaceae)Seeds.” Eur. Bull. Drug Res., 13, 2005, 31-34.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









