 About Authors:
About Authors:
Kapil Sharma1*, Subhash Gupta 2, Yogesh Sharma1
1 Yaresun Pharmaceuticals Pvt. Ltd.Jaipur - 302006, Rajasthan, India.
2 Oasis test house ltd.jaipur-302006,
Rajasthan, India.
SPECTROPHOTOMETRIC SIMULTANEOUS ESTIMATION OF SPIRONOLACTONE AND TORSEMIDE IN COMBINED TABLET DOSAGE FORM USING BY MULTICOMPONENT MODE OF ANALYSIS
ABSTRACT
A method for simultaneous estimation of torsemide (TRS) and spironolactone (SPL) in combined tablet dosage form has been developed. The method employs the application of multicomponent mode of analysis. This method utilize 50 % v/v methanol in distilled water. TRS show maximum absorbance at a wavelength of 288 nm and SPL at 238 nm. Where the linearity ranges for TRS and SPL were 1-5µg/ml and 5-25 µg/ml respectively. The procedure was successfully applied for the simultaneous determination of both drugs in laboratory prepared mixture and in market available tablet dosage form. The accuracy of the method was assessed by recovery studies and was found to be 98.15±0.64 and 100.27±0.15 for TRS and SPL respectively. Results of the analysis were validated statistically so that it can be used for routine analysis of TRS and SPL in combined tablet dosage form.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1180
INTRODUCTION
Torsemide is chemically {3-isopropyl carbamyl sulphonamido-4-(31-methyl phenyl) amino pyridine} and used as diuretic. It inhibits Na+, K+, Cl- symport across the thick ascending limb of the loop of Henley. Spironolactone is chemically {(7α, 17α)-7-(Acetylthio)-17-hydroxyl-3-oxopregn-4-ene-21-carboxylic acid-γ-lactone} and used as diuretic. Spironolactone acts from the interstitial side of the tubular cell; combines with the mineralocorticoids receptor and inhibits the formation of aldosterone induced proteins (AIPs) in a competitive manner1. TRS is official in USP2 and SPL is official in USP3 and IP4. Literature survey reveals that TRS was determined by GC-MS5 and three HPLC6-8methods and SPL was determined by quantitative spectrophotometric method9-10 and HPLC methods10-12. No spectrophotometric method has been reported for the estimation of both these drugs in a combined tablet dosage form . The aim of this paper was to develop simultaneous spectrophotometric method for quantitative determination of TRS and SPL in combined tablet dosage form by using multicomponent mode of analysis. The results of analysis using the spectrophotometric method for estimation was found to be satisfactory such that the developed method can be used for routine analysis of drugs from tablet dosage form. The method is fast and economical.
MATERIAL AND METHOD
Instrument, reagents and chemicals
A dual-beam shimadzu UV-visible spectrophotometer 1601 was used. Freshly prepared 50 % v/v methanol (LR, Merck, Mumbai) in distilled water was used as solvent. Gift sample of TRS and SPL was procured from Micro labs ltd, Bangalore. Tablets of 10 mg TRS and 50 mg SPL strength were procured from local pharmacy of Retorlix-10 brand (Lupin, Mumbai).
Preparation of stock solution of torsemide
Stock solution of containing 500 µg/ml of torsemide was prepared by dissolving 25 mg torsemide in 50 ml 50 % v/v methanol in distilled water.
[adsense:468x15:2204050025]
Preparation of stock solution of spironolactone
Stock solution containing 500 µg/ml of spironolactone was prepared by dissolving 25 mg spironolactone in 50 ml 50 % v/v methanol in distilled water.
Preparation of laboratory synthetic mixture of torsemide and spironolactone
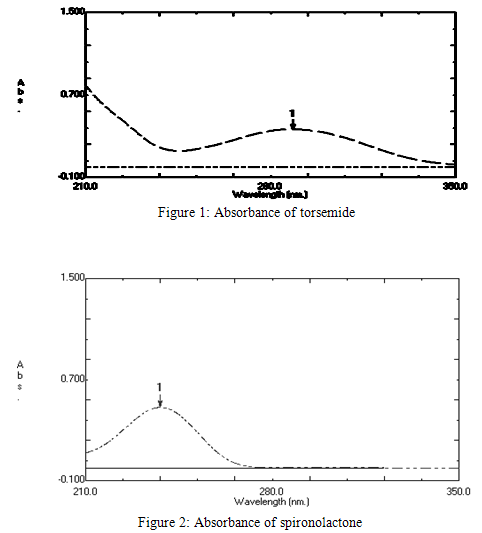
Laboratory synthetic mixture of TRS and SPL were prepared in the ratio of 1:5 respectively. This decision is based on the combination of TRS and SPL available in the combined tablet dosage form. Laboratory synthetic mixture of TRS and SPL were prepared in the ratio of 10:50 respectively was prepared by dissolving 1 ml of TRS stock solution and 5 ml SPL stock solution in to 50 ml 50 % v/v methanol in distilled water. Maximum absorbance was found to be at 288 nm and 238 nm for TRS and SPL respectively. The spectrums are shown in figure 1 and 2. Overlay spectrum is shown in figure 3.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Linearity and accuracy
Linearity and accuracy in the concentration range of 1-5 mg/ml for TRS and 5-25 mg/ml for SPL were examined employing intraday and interday studies for the determination of TRS and SPL. The results for intraday and interday experiments with a good correlation were obtained and evaluated statistically as demonstrated in table 1 and 2. Calibration curve is shown in figure 4 and 5. Optical parameters and Regression characteristics are shown in table 3.
Table 1: Recovery study of torsemide and spironolactone (Retorlix-10)
|
S.No |
Drug |
Conc. before spiking (mg/ml) |
Reference Std. added (mg/ml) |
Conc. after spiking (mg/ml) |
percent Recovery |
Mean percent Recovery |
|
1 |
Torsemide |
0.998 |
0.996 |
1.98 |
98.40 |
98.15 ± 0.64 |
|
0.998 |
1.992 |
2.98 |
99.59 |
|||
|
0.998 |
2.988 |
3.97 |
99.42 |
|||
|
2 |
Spironolactone |
4.98 |
5 |
9.99 |
100.22 |
100.27 ± 0.15 |
|
4.98 |
10 |
15.01 |
100.37 |
|||
|
4.98 |
15 |
19.99 |
100.06 |
Table 2: Summary of Validation Parameters
|
PARAMETER |
OBSERVATION |
|
|
Torsemide |
Spironolactone |
|
|
Linearity (Correlation coefficient r2) |
0.9998 |
0.9999 |
|
Accuracy (% Recovery) |
98.15% ± 0.64 |
100.27% ± 0.15 |
|
Precision RSD Repeatability (n= 6) Intra-day (n=3) Inter-day (days=3) |
0.465 0.788 0.954 |
0.090 0.037 0.077 |
Table 3: Optical parameters and Regression characteristics of torsemide and spironolactone
|
Parameters |
Torsemide |
Spironolactone |
|
288 nm |
238 nm |
|
|
Beer’s law limit (mg/ml) |
0-5 |
0-25 |
|
Molar absorptivity (l mole-1cm-1) |
1.35 x 105 |
1.99 x 105 |
|
Sandell's sensitivity (mg/cm2/0.001absorbance unit) |
2.58 x 10-3 |
2.09 x 10-3 |
|
Regression equation |
0.0381 |
0.0475 |
|
Correlation coefficient (r) |
0.9985 |
0.9998 |
Application of the proposed procedure for the estimation of torsemide and spironolactone in combined tablet dosage form
Twenty tablets were taken, and accurately weighed. The tablets were crushed to a fine powder. The powder sample equivalent to 10 mg of torsemide and equivalent 50 mg of spironolactone was transferred to a 100 ml volumetric flask and about 80 ml of 50 % v/v methanol in distilled water was added and sonicated to dissolve. The volume was made up to the mark with 50 % v/v methanol in distilled water. This solution was filtered through whatman filter paper 42. This solution (10 ml) was diluted to 100 ml with 50 % v/v methanol in distilled water. The solutions were analyzed by multicomponent mode of analysis. As blank 50 % v/v methanol in distilled water was used.
RESULTS AND DISCUSSIONS
A UV-spectrophotometric (multicomponent mode of analysis) method was developed for the estimation of torsemide and spironolactone in combined tablet dosage form. Solvent used was 50 % v/v methanol in distilled water. Measurement was done at 288 nm and 238 nm for torsemide and spironolactone respectively. Beer’s law was obeyed in the concentration range of 1-5 mg/ml for torsemide and 5-25 mg/ml for spironolactone. The value of standard deviation was satisfactory and the recovery studies were 98.15%± 0.64 for TRS and 100.27%±0.15 for SPL. The value of relative standard deviation in repeatability study was found 0.465 % for torsemide and 0.090 % for spironolactone. In intra-day precision value of relative standard deviation was found 0.788 % for torsemide and 0.037 % for spironolactone. The value of relative standard deviation for inter-day precision was found 0.954 % for torsemide and 0.077 % for spironolactone. Hence the newly developed method can be used for routine analysis for the estimation of torsemide and spironolactone in combined tablet dosage form.
REFERENCES
1. CIMS, Current index of medical specialties, Bangalore: CMP Media India Pvt. Ltd: 2006.
2. United states pharmacopoeia, 28th ed, Washington, Published by the board of trustees, 2005:Vol-II: 1799-1800.
3. United states pharmacopoeia, 28th ed, Washington, Published by the board of trustees, 2005:Vol-II: 1953.
4. Indian Pharmacopoeia, 4th ed, New Delhi India, the controller of publications: 1996: Vol-II:709-710.
5. Barroso MB, Meiring HD, jong A.de, Alonso RM, Jimenez RM. Gas chromatographic-mass spectrometric analysis of the loop diuretic torsemide in human urine. J Chromatogr B Biomed Sci Appl 1997;690:105-13.
6. March C, Farthing D, Well B, Besenfelder E, Karnes HT. Solid-phase extraction and liquid chromatographiy of torsemide and metabolites from plasma and urine. J Pharm Sci 1990; 79:453-7.
7. Barroso MB, Alonso RM, Jimenez RM. Simultaneous determination of torsemide and its major metabolite M5 in human urine by high-performance liquid chromatography-electrochemical detection. J Chromatogr Sci 2001; 39; 491-6.
8. Engelhardt S, Meineke I, Brockmoller J. Improved solid- phase extraction and HPLC measurement of torsemide and its important metabolites. J Chromatogr B Analyt Technol Biomed life Sci 2006;831:31-5
9. Dinc Erdal, Ustunda Ozgur. Spectrophotometric quantitative resolution of hydrochlorothiazide and spironolactone in tablets by chemometric analysis method. II Farmaco 2003; 58:1151-61.
10. Martin E, Jimenez AI, Hernandez O, Jimenez F,Arias JJ. Simultaneous kinetic spectrometric determination of spironolactone and canrenone in urine using partial least-square regression.Talanta 1999; 49:143-54.
11. De Croo F, Bossche W. vanden, Moerloose P.de. Simultaneous determination of spironolactone and althiazide in tablets by high-performance liquid chromatography. Fresenius’ Journal of Analytical chemistry 2004; 323:91-93.
12. Kenneth S. Alexander, Shyam Sunder K.S. Vangala, David Dollimore. Improved High-performance liquid Chromatography Assay for spironolactone analysis.Drug development and industrial pharmacy 1998; 24:101-7.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









