 About Authors: Deepak Sahu
About Authors: Deepak Sahu
M.Pharm, Geetanjali Institute of Pharmacy
Dabak,
Udaipur, Rajasthan
ABSTRACT
A simple, accurate, low cost and specific HPTLC method for estimation of quetiapine fumarate in tablet has been developed. It was performed on Silica gel G60 F254 aluminium foil using acetonitrile : chloroform in the ratio of 1:9 as mobile phase. The mobile phase having chamber was saturated for 20 minutes at room temperature. The Rf value of quetiapine fumarate was found to be 0.45. The plate was scanned and quantified at 242 nm. The calibration curve response was observed between 5-25 μg. The linear regression data showed good linear relationship of r = 0.999. The percent recovery was found to be 100.0 ± 0.01.The developed method was validated for its accuracy and precision with suitable parameters.
[adsense:336x280:8701650588]
INTRODUCTION
Quetiapine is indicated for the treatment of schizophrenia, depressive episodes associated with bipolar disorder, acute manic episodes associated with bipolar I disorder (as either monotherapy or adjunct therapy to lithium or valproate), and maintenance treatment of bipolar I disorder (as adjunct therapy to lithium or divalproex). Quetiapine received its initial indication from the U.S. Food and Drug Administration for treatment of schizophrenia & mania-associated bipolar disorder. It is sometimes used off-label, often as an augmentation agent, to treat conditions such as obsessive-compulsive disorder, post-traumatic stress disorder, restless legs syndrome, autism, alcoholism, depression, Tourette syndrome, and has been used by physicians as a sedative for those with sleep disorders or anxiety disorders.
Quetiapine is a dopamine, serotonin and adrenergic antagonist and a potent antihistamine with clinically negligible anticholinergic properties. Quetiapine binds strongly to serotonin receptors. Serial PET scans evaluating the D2 receptor occupancy of quetiapine have demonstrated that quetiapine very rapidly disassociates from the D2 receptor. Theoretically, this allows for normal physiological surges of dopamine to elicit normal effects in areas such as the nigrostriatal and tuberoinfundibular pathways, thus minimizing the risk of side effects such as pseudo-parkinsonism as well as elevations in prolactin. Quetiapine is partly metabolized to Norquetiapine which also acts as a potent noradrenaline reuptake inhibitor and muscarinic antagonist.
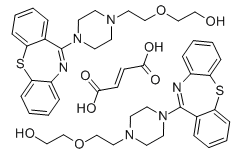
From the literature review many analytical methods2,3,4,5 have been reported for the determination of quetiapine fumarate such as spectrophotometry, HPLC. There is no reported HPTLC method for the determination of quetiapine fumarate in tablet dosage form. The objective of this work is report a simple, precise, accurate and cost effective HPTLC method for estimation of quetiapine fumarate is quantified at 242 nm.
MATERIALS AND METHODS
A Camag, Linomat 5 sample applicator was used. The scanner used was Camag TLC Scanner 3 and CATS 4 software for interpretation of data. Quetiapine fumarate standard pure drug was supplied by M/S IPCA Laboratories, Ratlam (M.P.). Acetonitrile and Chloroform used were of AR grade purchased from S.D Fine Chemicals Ltd. All other chemicals used in the analysis were AR grade.
Standard preparation
Accurately weighed 10 mg of quetiapine fumarate was transferred into10 ml volumetric flask; methanol was added to dissolve and made up to mark with the same (1μg/1μl).
Chromatographic conditions
Stationary phase-silica gel G60F254 TLC precoated plates (10*10), Mobile phase acetonitrile : chloroform in ratio of 1:9, saturation time - 20 Minutes, Migration distance - 56 cm, Band width - 6mm, Source of radiation - Deuterium lamp, Detection wavelength - 242nm using slit dimension 5x6.5 mm.
Calibration curve response
Aliquots of 5, 10, 15, 20 and 25 μl of standard solution of quetiapine fumarate were applied on the chromatographic plates. The plate was developed using acetonitrile: chloroform (1:9) dried and scanned at 242 nm between peak areas/concentration was observed for quetiapine fumarate.
Sample preparation
Twenty tablets were taken and average weight was calculated. The content of quetiapine fumarate was weighed equivalent to 100 mg and it was taken in a 50 ml volumetric flask and dissolved with small portion of methanol. The solution was shaken well and filtered through whattman filter paper then the volume was made up to mark using methanol.
Assay
From the sample solution aliquots was spotted (10 μl and 15 μl) on the plate by using Linomat 5 applicator. Developed chromatogram was scanned. A triplicate of those was carried out the peak areas were noted and the amount present formulation was calculated using standard calibration curve. The result of assay is displayed in Table 1.
Recovery study
To study the accuracy and precision of the method recovery experiment to determine if there are positive or negative interferences from excipients present in formulation. The recovery of added standard was studied at 3 different levels, each being analyzed in a manner similar to described for assay. Each set of addition was reported seven times and the recovery of added standard was calculated.
Table 1: Analysis for formulation
|
S. No |
Drug |
Label claim (mg/Tablet) |
Assay |
% Label claim |
|
1 |
Quetiapine Fumarate |
100 |
99.9 ± 0.05 |
99.5 ± 0.04 |
Validation
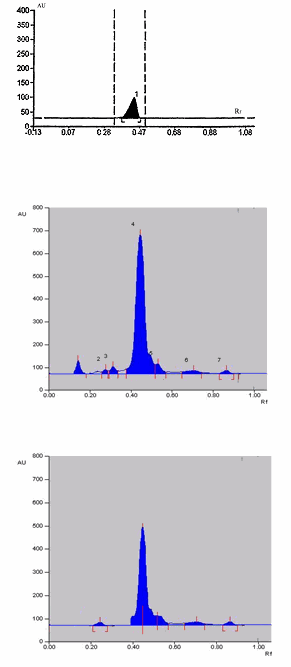
The developed method was validated as per ICH guidelines for specificity and accuracy (Table 2). The method is found to be specific for quetiapine fumarate since it resoled the peak (Rf = 0.45) in presence of other excipients in the formulation (Fig.1). The correlation co-efficient (r) and other validation parameters are given in Table2. The precision of method was studied by Intra-day and Inter-day assays. The %RSD was found to be 0.815 and 0.860 respectively. Precision of the instrument was checked by repeated scanning of two different concentrations for 3 times on the same day. Accuracy of the method was developed by carrying out recovery studies.
Repeatability of the method was checked by analyzing a standard solution of Quetiapine fumarate after application on a TLC plate.
Table 2: Validation Parameters
|
Parameter |
Quetiapine Fumarate |
|
Precision: |
|
|
Intra-day (%RSD) |
0.815 |
|
Inter-day (%RSD) |
0.860 |
|
Repeatability |
0.005 |
|
Specificity |
Specific |
|
Linearity range |
5-25 μg/spot |
|
Correlation co-efficient (r) |
0.999 |
Fig. 1: Chromatogram for quetiapine fumarate in tablet dosage form
RESULTS AND DISCUSSION
The developed method was precise and drug is resolved in well chromatographic system. From the standard deviation, it was observed that the method was precise. The content of Quetiapine fumarate was found to be 99.9 ± 0.05 and the percent recovery 100.0 ± 0.01 using precoated silica gel G60F254 on aluminium foil and a mobile phase comprising acetonitrile: chloroform (1:9) which gives good separation of Quetiapine fumarate ( Rf = 0.45). The result of assay is displayed in Table 1.
The detector response of Quetiapine fumarate was found to be linear in the range of 5-25 μg/spot. The correlation co-efficient obtain for the linearity Quetiapine fumarate was 0.999. The result of assay is displayed in Table 2. Low standard deviation indicated that the present method is more accurate, so the method can be used for routine analysis of quetiapine fumarate in dosage forms (Fig.1).
CONCLUSION
There are several methods existing for the estimation of Quetiapine fumarate viz HPLC, spectrophotometry. These methods are either costlier or cannot detect impurity whereas the HPTLC method developed can simultaneously run standards and formulation. Therefore it is concluded that the HPTLC method is cost effective, less time consuming, precise and accurate.
REFERENCES
1. Bhudarari S: Eds, In: The Merck Index, 13th edition, Merck and co., 4241; PageNo.741.
2. Prabhakar A H, Patel V B, Girighar: Spectrophotometric determination of quetiapine fumarate hydrochloride in bulk and in pharmaceutical formulations. J Pharm Biomed Anal 1999; 20 (3): 427-32.
3. Erturk S, Cetin S M, Atmaca S, Ersoy L, Baktr G: A sensitive HPLC method for the determination of quetiapine fumarate and norquetiapine fumarate in human plasma with fluorescence detection. Ther Drug Monit 2005; Feb 27 (1): 38-43.
4. Mandrioli R, Pucci V, Visini D, Varani G, Raggi M A: Rapid methods for determination of quetiapine fumarate in pharmaceutical formulations. J Pharm Biomed Anal 2002; Aug 29 (6): 1127-34.
5. Cherkaoui S, Veuthey J L: Nonaqueous capillary electrophoresis - electrospray-mass spectrometry for the analysis of quetiapine fumarate and its related compounds. Electrophoresis 2002 Feb; 23 (3): 442-448.
Reference ID: PHARMATUTOR-ART-1018
FIND OUT MORE ARTICLES AT OUR DATABASE









