 About Authors:
About Authors:
YASWANTH ALLAMNENI1*, PAVAN POTTURI1, RAJKUMAR NULU2, RAVADA RAMESH3, P DAYANANDA CHARY1, ARUN KALEKAR1.
1Research and Development Department, Natco Pharma Limited, Kothur, Mahaboobnagar,Andhra Pradesh, India-509228.
2Research and Development Department, Aurobindo Pharma Ltd., Hyderabad, Andhra Pradesh, India.
3HOD, Dept. of Pharmaceutics, Dr. H.L.T. College of pharmacy, Kengal, Channapatna, Banglore(R).
*yaswanthallamneni@gmail.com
ABSTRACT
The aim of this study was to investigate the influence of superdisintegrants on the dissolution properties of a poorly water-soluble drug from complexes prepared by kneading method. Ketoprofen was employed as a model drug. Solid dispersions prepared by kneading method exhibited higher dissolution rate and DE30 values in each case. Ketoprofen tablets were prepared by direct compression technique using microcrystalline cellulose as a directly compressible vehicle. The formulated tablets were evaluated by different In Process Quality Control tests, content uniformity and in vitro drug release study. The FTIR spectra’s study revealed that there were no interaction between polymers and drug. All the tablets formulated employing solid dispersions in superdisintegrants gave rapid and higher dissolution of Ketoprofen when compared to that of Ketoprofen plain tablets. Ketoprofen dissolution from all the tablets followed first order kinetic with correlation coefficient ‘r’ above 0.9470. All dissolution parameters (k1, DE30 and T30) indicated rapid and higher dissolution of Ketoprofen from tablets formulated employing its solid dispersions in superdisintegrants when compared to plain tablets.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1273
INTRODUCTION
Oral route has been one of the most popular routes of drug delivery due to its ease of administration, patient compliance, least sterility constraints and flexible design of dosage forms. Systemic drug absorption from a drug product consists of a succession of rate process for solid oral, immediate release drug products1. The rate process includes dissolution of the drug in an aqueous environment and absorption across cell membranes into systemic circulation.
For drugs that have very poor aqueous solubility, the rate at which the drug dissolves (dissolution) is often the slowest step and therefore exhibits a rate limiting effect on drug bioavailability. In contrast, for a drug that has a high aqueous solubility the dissolution rate is rapid the rate at which the drug crosses or permeates cell membrane is the slowest or rate limiting step. The main possibilities for improving dissolution according to modified Noyes – Whitney analysis2 are to increase the surface area available for dissolution by decreasing the particle size of the solid compound and/or by optimizing the wetting characteristics of the compound to decrease the boundary layer thickness, to ensure sink conditions for dissolution and, last but definitely not least, to improve the apparent solubility of the drug under physiologically relevant conditions.
Solid dispersion3 is a unique approach to present a poorly soluble drug an extremely fine state of subdivision gastro intestinal fluids. This dispersion consists of a micro crystalline dispersion of a poorly soluble drug in matrix consisting of physiologically inert, readily soluble carrier. Exposure of this type of solid dispersion to the gastro intestinal fluids results in water soluble carrier and exposes the dispersed poorly soluble drug. The solubility characteristics of the drug may be altered by reduction practical size. Several insoluble drugs have been shown to improve their dissolution character when incorporated into solid dispersion. It releases the drug through different mechanisms, and the rate of release of drug to the surrounding fluid is mainly dependent on the type of solid dispersion formed. Solid dispersion technique has been widely employed to improve the dissolution rate, solubility and oral absorption of poorly water soluble drugs.
Ketoprofen is non steroidal anti-inflammatory drug, which is used in the prevention and treatment of rheumatoid arthritis and osteoarthritis. Ketoprofen belongs to class II drug in BCS classification i.e. low solubility and high permeability. One of the major problems with this drug is its low solubility in biological fluids, which results into poor bioavailability after oral administration.
In Kneading method, the physical mixture of drug and carrier were triturated using small quantity of organic solvent and water mixture3, usually alcohol and water (1:1 v/v).The slurry is kneaded for 45 minutes and dried at 45 degrees Celsius. The dried mass is pulverized and sieved through sieve no 60 and the fraction was collected. The advantages of this method are low temperature requirement for solid dispersion preparation and usage of organic solvent is less. This method of preparation avoids thermal degradation of drug and employs less quantity of organic solvent.
As day’s passes, demand for faster disintegrating formulation is increased. So, pharmacist needs to formulate disintegrants i.e. Superdisintegrants4 which are effective at low concentration and have greater disintegrating efficiency and they are more effective intragranularly and this superdisintegrants act by swelling and due to swelling pressure exerted in the outer direction or radial direction, it causes tablet to burst or the accelerated absorption of water leading to an enormous increase in the volume of granules to promote disintegration.
Microcrystalline cellulose is widely used in pharmaceuticals, primarily as a binder/diluent in oral tablet and capsule formulations where it is used in both wet-granulation and direct-compression processes5. In addition to its use as a binder/diluent, microcrystalline cellulose also has some lubricant and disintegrant properties that make it useful in tableting. Several different grades of microcrystalline cellulose are commercially available that differ in their method of manufacture, particle size, moisture, flow, and other physical properties. The larger particle size grades generally provide better flow properties in pharmaceutical machinery. Low-moisture grades are used with moisture-sensitive materials. Higher-density grades have improved flowability.
Lactose is widely used as a filler and diluent in tablets and capsules, and to a more limited extent in lyophilized products and infant formulas. Usually, fine grades of lactose are used in the preparation of tablets by the wet-granulation method or when milling during processing is carried out, since the fine size allows better mixing with other formulation ingredients and utilizes the binder more efficiently.
Magnesium stearate is widely used in cosmetics, foods, and pharmaceutical formulations. It is primarily used as a lubricant in capsule and tablet manufacture at concentrations between 0.25% and 5.0% w/w. It is also used in barrier creams. Magnesium stearate is hydrophobic and may retard the dissolution of a drug from a solid dosage form; the lowest possible concentration is therefore used in such formulations.Talc was once widely used in oral solid dosage formulations as a lubricant and diluents.
MATERIALS AND METHODS
[adsense:468x15:2204050025]
Materials
Ketoprofenwas provided by Natco Pharma Ltd., Hyderabad, India. Primojel, Crospovidone, Croscarmellose and Magnesium stearate were purchased from Signet Chemical Carporation, Mumbai, India. Microcrystalline cellulose was purchased from FMC Biopolymer, Japan and Talc was purchased from Luzenal Valchisone, Italy. Methanol was purchased from Merck Specialities Pvt. Ltd., Mumbai, India and other reagents were of analytical grade.
Methods
Preparation of Solid Dispersions With Superdisintegrants6
In kneading method, carriers i.e. Primojel, Crospovidone and Croscarmellose in different ratios were taken into a mortar along with ketoprofen with a small volume of a solvent of water- methanol (3:2). The thick slurry was kneaded for 45 min and then dried at 550c until dry. The dried mass was pulverized and sieved through mesh 100#.
Evaluation of Ketoprofen Solid Dispersions in superdisintegrants
a.In vitrodissolution study
The prepared solid dispersions were subjected to in vitro dissolution. Dissolution test was carried out using USP Tablet dissolution apparatus type II. The stirring rate was 50 rpm, pH-7.4 phosphate buffer was used as dissolution medium and dissolution medium was maintained at 37±1oC. Samples of 5 ml were withdrawn at regular intervals of time, filtered and replaced with 5 ml of fresh dissolution medium, dilutions were made wherever necessary and were analyzed for Ketoprofen at 260.5 nm by using UV-visible spectrophotometer.
b. Estimation of Drug Content
100 mg of solid dispersion was taken in a 50 ml volumetric flask. Methanol about 40 ml was added and mixed thoroughly. The contents were repeatedly warmed in a hot bath while mixing to dissolve the drug in the solvent. The solution was made up to volume with methanol. The solution was then suitably diluted and assayed for drug content by the specific spectrophotometric method.
c. Fourier Transform – Spectroscopy
The infrared (IR) spectra were recorded using an FTIR spectrophotometer (Perkin Elmer Spectrum GX) by the KBr pellet method in the wavelength region between 4000 cm-1 and 400 cm-1. The spectra obtained for Ketoprofen and physical mixtures of Ketoprofen with polymers were compared to check compatibility of drug with polymers.
Formulation of Ketoprofen Tablets By Direct Compression Method8
Ketoprofen Tablets were prepared by direct compression technique. Accurately weighed quantities of Ketoprofen/Ketoprofen solid dispersions (Ketoprofen and different superdisintegrants in ratio of 1:3), Microcrystalline cellulose and Lactose were passed through sieve no. 40# and homogeneously blended. To the above blend sifted (40#) magnesium stearate and talc were added and homogeneously blended. Finally lubricated blend was evaluated for flow properties like Angle of repose, Bulk Density, Tapped density, Hausner’s ratio and Carr’s index value. The Lubricated blend was compressed in to tablets by using Mini Press II (Rimek) compression machine.
All the formulations i.e. tablets containing Ketoprofen and Ketoprofen solid dispersions were evaluated for their drug content uniformity, thickness, hardness, friability and drug release.
Punch Description:10.00 mm Round shaped punches embossed with "50" on one side and plain surface on other side.
Preformulation testing7
Preformulation testing is the first step in the rational development of dosage forms of a drug substance. It can be defined as an investigation of physical and chemical properties of a drug substance alone and when combined with excipients. Overall objective of preformulation testing is to generate information useful to the formulator in developing stable and bio-available dosage forms.
Preformulation tesing need to be performed on lubricated blend of all batches like angle of repose, Bulk Density, Tapped density, Hausner’s ratio and Compressibility Index Value.
tan θ = h / r
θ = tan-1 (h / r)
Where, h = height of pile, r = radius of the base of the pile, θ = angle of repose.
Bulk Density = (Weight of the Blend / Bulk volume)
Tapped Density = (Weight of the Blend / Tapped volume)
Carr’s Index (%) = [(Tapped density – Bulk Density) / Tapped Density] × 100
Hausner’s Ratio = Tapped density / Bulk Density
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Evaluation of Tablets8
a. Weight variation test
This test is performed to maintain the uniformity of weight of each tablet, which should be in the prescribed range. 20 tablets were selected randomly and individual weight as well as average weights was taken. Not more than two of the individual weights deviate from the average weight by more than the percentage shown in the following table and none deviate by more than twice the percentage. The mean and standard deviation were determined.
b. Hardness
Hardness of tablets was measured by using Hardness tester. (LabIndia TH 1050S Automatic Tablet Hardness Tester).
c. Thickness
Thickness of tablets was measured by using Digital Vernier Calipers. (Mitutoyo).
d. Disintegration Time
Disintegration time was measured by using Disintegration Tester (Electrolab ED-2AL).
e. Friability
Friability test was performed with Friabilty test Apparatus (Electrolab EF-2 Friabilator) and the Limit for % loss is Not More Than 1.0%w/w.
f. Drug Content
From each batch of prepared tablets, ten tablets were collected randomly and powdered. 100 mg of powder, which was equivalent to 50 mg of drug, was accurately weighed and transferred to 100ml volumetric flask. Then, the volume was made up with pH-7.4 phosphate buffer and shaken for 10 min to ensure complete solubility of the drug. Then the solution was filtered. Same concentration of standard solution was prepared by dissolving 10 mg of standard drug in PH-7.4 phosphate buffer. For both the sample and standard solutions absorbance was measured at 260.5 nm in UV-Visible spectrophotometer.
g. In-VitroDrug Release Studies
The release rate of Ketoprofen from Tablets was determined using USP XXIV (model DISSO, M/s Labindia) rotating paddle method (Apparatus II). The dissolution test was performed using 900ml of 7.4 pH Phosphate buffer, 37 ±0.5°C at 50 RPM. 5 ml sample was withdrawn at different intervals and replaced with fresh medium. The samples were filtered through Whatman filter paper no. 41.After suitable dilution solution’s absorbance was measured at 260.5 nm using a double beam UV spectrophotometer (Shimadzu 1700).
h. Dissolution Efficiency9
Another parameter suitable for the evaluation of in vitro dissolution has been suggested by Khan who introduced the parameter Dissolution Efficiency (DE). DE is defined as the area under the dissolution curve upto a certain time ‘t’ expressed as a percentage of the area of the rectangle described by 100% dissolution in the same time.
Dissolution Efficiency (DE) =
The dissolution efficiency can have a range of values depending on the time intervals chosen. In any case, constant time intervals should be chosen for comparison. For example, the index DE30 would relate to the dissolution of the drug from a particular formulation after 30 minutes and could only be compared with DE30 of other formulations. Summation of the large dissolution data into a single figure DE enables ready comparison to be made between a large number of formulations.
i. In-VitroDrug Release Kinetics10
The dissolution data were subjected to release kinetic study. Drug Release data were fitted to kinetic model including the Zero order, First order release equations to find the equation with the best fit.
RESULTS AND DISCUSSION
In the present study, kneading method was employed to prepare solid dispersions. In this case, solid dispersions were prepared at 1:1, 1:2 and 1:3 ratios of drug and different superdisintegrants (F1, F2, F3 were 1:1, 1:2, 1:3 ratios respectively for primojel, F4, F5, F6 were 1:1, 1:2, 1:3 ratios respectively for crospovidone and F7, F8, F9 were 1:1, 1:2, 1:3 ratios respectively for croscarmellose). The solid dispersions prepared were evaluated for drug content uniformity, dissolution rate and dissolution efficiency. Drug contents in various solid dispersions were found to be within 100±5 % of the theoretical amount. In each case low CV values (< 1.5%) in the percent drug content indicated uniformity of drug content in the solid dispersions prepared.
FT-IR spectrum of pure ketoprofen was shown in Figure 1. The characteristic peaks are 1695 due to C=O stretching of the acid, 1655 due to C=O stretching of the ketone and 3200-2500 due to O-H stretching. FT-IR spectrum of ketoprofen with Primojel (sodium starch glycollate) was shown in Figure 2. The characteristic peaks are 1697 due to C=O stretching of the acid, 1655 due to C=O stretching of the ketone and 2937 due to O-H stretching. FT-IR spectrum of ketoprofen with crospovidone was shown in Figure 3. The characteristic peaks are 1696 due to C=O stretching of the acid, 1655 due to C=O stretching of the ketone and 2925 due to O-H stretching. FT-IR spectrum of ketoprofen with croscarmellose was shown in Figure 4.
The characteristic peaks are 1697 due to C=O stretching of the acid, 1655 due to C=O stretching of the ketone and 2924 due to O-H stretching. Solid dispersion of superdisintegrants with ketoprofen exhibited higher dissolution rates and dissolution efficiency values than the corresponding pure drug. The feasibility of formulating these dispersions into tablet dosage forms is evaluated. Solid dispersions prepared by kneading method were formulated into tablets as these dispersions exhibited higher dissolution rates.
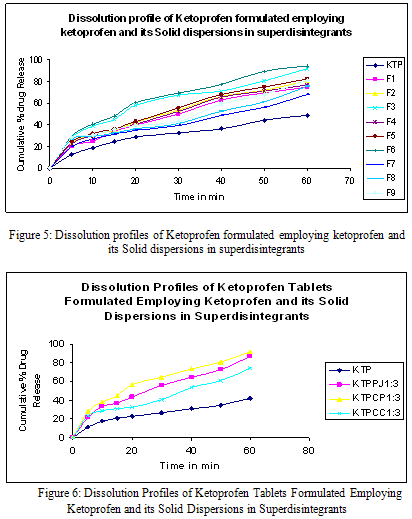
The dissolution rate Ketoprofen from superdisintegrants solid dispersions were studied in phosphate buffer of pH-7.4 and compared with that of pure drug. The correlation coefficient values in the analysis of dissolution data as per zero order, first order kinetics, higuchi kinetics and korsemeyer-peppas model are determined. The correlation coefficient values were found to be relatively higher in the case of first order model in all the cases. Thus the dissolution of ketoprofen as such and from various ratios of different superdisintegrants followed first order kinetics. Solid dispersions prepared by kneading method exhibited higher dissolution rate and DE30 values in each case. A 3.59, 4.46 and 2.39 folds increase in the dissolution rate of ketoprofen was observed with Primojel, crospovidone and Croscarmellose (1:3) respectively prepared by kneading method.
Thus both the solubility and dissolution rates of ketoprofen were markedly enhanced by dispersion in superdisintegrants. Solid dispersion of superdisintegrants with ketoprofen exhibited higher dissolution rates and dissolution efficiency values than the corresponding pure drug. The feasibility of formulating these dispersions into tablet dosage form is evaluated.
Ketoprofen (50 mg) tablets were formulated employing different superdisintegrants (1:3) kneaded dispersions by direct compression method. All the solid dispersion tablets prepared were found to contain the drug within 100±3% of the labeled claim. Hardness of the tablets was in the range of 9.0-12.0 kp and was satisfactory. Friability of Ketoprofen tablets wasfound to be less than 1%. The thickness of the all tablets wasfound to be in the range of 4.60 to 4.80 mm. All the tablets prepared by direct compression method disintegrated rapidly within 4 min.
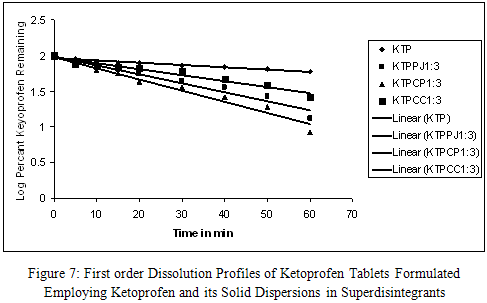
All the tablets formulated employing solid dispersions in superdisintegrants gave rapid and higher dissolution of ketoprofen when compared to that of ketoprofen plain tablets. Ketoprofen dissolution from all the tablets followed first order kinetic with correlation coefficient ‘r’ above 0.9470. All dissolution parameters (k1, DE30 and T30) indicated rapid and higher dissolution of ketoprofen from tablets formulated employing its solid dispersions in superdisintegrants when compared to plain tablets (KTP).
A 3.71, 4.62 and 2.44 fold increase in the dissolution rate was observed respectively with formulations KTPPJ 1:3, KTPCP 1:3 and KTPCC 1:3 when compared to plain tablets, KTP. The increasing order of dissolution rate of ketoprofen from tablets observed with various superdisintegrants was CP>PJ>CC>PLAIN KETOPROFEN TABLETS.
CONCLUSION
The present work has demonstrated that the preparation of ternary solid dispersion of Ketoprofen with superdisintegrants by using Kneading method considerably improves the dissolution rate of ketoprofen from powdered forms and tablets. Solid dispersions prepared by kneading method exhibited higher dissolution rate and DE30 values in each case. The FTIR spectra’s revealed that there were no interaction between polymers and drug. It could be concluded that the superdisintegrants can be used for enhancement of dissolution of Ketoprofen.
REFERENCES
1. Christian Leuner., Jennifer Dressman. Improving drug solubility for oral delivery using solid dispersions. European journal of pharmaceutics and biopharmaceutics., 2000, 50: 47-60.
2. Leon Shargel., Andrew B.C. Applied biopharmaceutics and pharmacokinetics. Appleton-Century-Crofts., 4th Ed, 1985, 134- 136.
3. NagasamyVenkatesh D., Sangeetha S., karthick S., Mohammed K., Fakruddin, Vivek G., Samantha M.K, shankar S., Elango K. Solid dispersions: A unique technique to imrove the aqueous solubility of poorly soluble drugs- A Review. International journal of pharma research., 2008, 05-12.
4. Zhao N., Augsburger LI. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorthiazide from directly compressed tablets. AAPS Pharm Sci Tech., 2005, 6(1): E120–E126.
5. Lerk CF et al. Effect of microcrystalline cellulose on liquid penetration in and disintegration of directly compressed tablets. J Pharm Sci., 1979, 68: 205–211.
6. Aftab modi., pralhad Tayade. Enhancement of dissolution profile of solid dispersion (Kneading) technique. AAPS PharmsciTech., 2006, 7(3): E1-E6.
7. Martin A., Swarbrick J., Cammarata A. Physical pharmacy. 3rd ed. Mumbai; K.M. Vargesh Company, 1991, Vol II: 620 – 627.
8. Adel M.Aly., M. Semreen., Mazen K.Qato. Superdisintegrants for solid dispersion to produce rapidly disintegrating Tenoxicam Tablets via camphor sublimation. Pharmaceutical Technology., 2005, 68-78.
9. Khan, K. A., J. Pharm. Pharmacol., 1975, 27: 48- 49.
10. Higuchi T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J Pharm Sci., 1963, 52: 1145-49.
11. Costa P. Sousa Lobo JM. Modeling and Comparison of Dissolution Profiles. Eur J of Pharm Sci., 2001, 13:123-33.
Table 1
Ketoprofen Solid Dispersions with Superdisintegrants
|
Sl.No |
Ingredient |
Ratio of Drug: Carrier |
||||||||
|
Batch No. |
||||||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
||
|
1. |
Ketoprofen |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
2. |
Primojel |
1 |
2 |
3 |
- |
- |
- |
- |
- |
- |
|
3. |
Crospovidone |
- |
- |
- |
1 |
2 |
3 |
- |
- |
- |
|
4. |
Croscarmellose |
- |
- |
- |
- |
- |
- |
1 |
2 |
3 |
Table 2
Ketoprofen Tablets Prepared Employing its Solid Dispersions in Superdisintegrants
|
Sl.No |
Ingredient |
Qty. per tablet (mg) |
|||
|
1. |
Ketoprofen |
50 |
- |
- |
- |
|
2. |
KTP-PJ 1:3 |
- |
200 |
- |
- |
|
3. |
KTP-CP 1:3 |
- |
- |
200 |
- |
|
4. |
KTP-CC 1:3 |
- |
- |
- |
200 |
|
5. |
Lactose |
181 |
31 |
31 |
31 |
|
6. |
MCC |
105 |
105 |
105 |
105 |
|
7. |
Talc |
7 |
7 |
7 |
7 |
|
8. |
Magnesium Stearate |
7 |
7 |
7 |
7 |
|
Total weight of the tablet (mg) |
350 |
350 |
350 |
350 |
|
Table 3
Flow properties of Lubricated Blend of all Formulations
|
Formulation Code |
Angle of repose (θ) (Mean±SD) |
Bulk Density (g/mL) (Mean±SD) |
Carr’s index (%) |
Hausner’s Ratio |
|
KTP |
24.1±0.2 |
0.610±0.08 |
14 |
1.172 |
|
KTPPJ 1:3 |
23.9±0.1 |
0.590±0.05 |
13 |
1.165 |
|
KTPCP 1:3 |
23.5±0.4 |
0.585±0.07 |
13 |
1.187 |
|
KTPCC 1:3 |
24.2±0.7 |
0.595±0.01 |
14 |
1.173 |
Table 4
Physicochemical Parameters Of Ketoprofen/Ketoprofen solid dispersion Tablets
|
Formulation Code |
Average weight of tablet (mg) |
Weight variation (%) |
Hardness (kp) |
Thickness (mm) |
Friability (%) |
Drug content (%) |
|
KTP |
351.5 |
-0.43 |
9.3 |
4.65 |
0.21 |
98.9 |
|
KTPPJ 1:3 |
350.3 |
-0.09 |
11.7 |
4.71 |
0. 15 |
99.6 |
|
KTPCP 1:3 |
349.6 |
0.11 |
10.3 |
4.68 |
0.17 |
99.4 |
|
KTPCC 1:3 |
350.5 |
-0.14 |
10.1 |
4.66 |
0.19 |
98.8 |
Table 5
In-VitroDrug Release Kinetics For Ketoprofen and Superdisintegrants Solid Dispersions
|
Formulation code |
Zero Order Model |
First-Order Model |
|
|
r2 |
r2 |
||
|
KTP (Pure Drug) |
0.9184 |
0.9600 |
|
|
F1 |
0.9457 |
0.9940 |
|
|
F2 |
0.9411 |
0.9884 |
|
|
F3 |
0.8852 |
0.9542 |
|
|
F4 |
0.9322 |
0.9912 |
|
|
F5 |
0.9384 |
0.9901 |
|
|
F6 |
0.8911 |
0.9747 |
|
|
F7 |
0.9272 |
0.9569 |
|
|
F8 |
0.9149 |
0.9287 |
|
|
F9 |
0.9246 |
0.9392 |
|
Table 6
Dissolution Parameters of Ketoprofen and Superdisintegrants Solid dispersions Prepared by Kneading Method in Phosphate Buffer of pH-7.4
|
Formulation code |
Dissolution Parameter |
|||
|
Drug content (%) |
T30 (min) |
DE30 (%) |
K1 (min-1) |
|
|
KTP (Pure Drug) |
- |
23.6 |
21.81 |
0.0099 |
|
F1 |
99.1 |
12.8 |
31.43 |
0.0223 |
|
F2 |
98.9 |
9.2 |
34.25 |
0.0253 |
|
F3 |
99.0 |
6.0 |
44.50 |
0.0356 |
|
F4 |
98.6 |
10.0 |
33.61 |
0.0232 |
|
F5 |
98.8 |
8.8 |
35.55 |
0.0274 |
|
F6 |
99.2 |
5.6 |
46.43 |
0.0442 |
|
F7 |
99.1 |
12.0 |
28.38 |
0.0161 |
|
F8 |
98.6 |
10.6 |
30.70 |
0.0191 |
|
F9 |
99.0 |
8.8 |
32.20 |
0.0237 |
Table 7
In-VitroDrug Release Kinetics For Ketoprofen And Solid Dispersions Tablets
|
Formulation code |
Zero Order Model |
First-Order Model |
|
r2 |
r2 |
|
|
KTP (Pure Drug) |
0.9163 |
0.9539 |
|
KTP |
0.9467 |
0.9556 |
|
KTPPJ 1:3 |
0.8998 |
0.9659 |
|
KTPCP 1:3 |
0.9345 |
0.9470 |
Table 8
Dissolution Parameters of Ketoprofen and Solid dispersions Tablets in Phosphate Buffer of pH-7.4
|
Formulation code |
Dissolution Parameter |
||
|
T30 (min) |
DE30 (%) |
K1 (min-1) |
|
|
KTP (Pure Drug) |
38.0 |
18.12 |
0.0078 |
|
KTP |
8.8 |
35.35 |
0.0290 |
|
KTPPJ 1:3 |
6.4 |
43.40 |
0.0361 |
|
KTPCP 1:3 |
14.2 |
28.68 |
0.0191 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









