About Authors:
SHARMA AKANSHA*, AGRAWAL SHIKHA
SWAMI VIVEKANAND COLLEGE OF PHARMACY
KHANDWA ROAD INDORE (M.P.)
*akansha.sharma@mylan.in
ABSTRACT :
The main aim for performing this project is to increase the bioavailabilty of lamotrigine as well as its onset of action to control the seizures that occur during the epileptic attack. To serve this purpose we will use mucilage of oscimum basilicum as a natural superdisintegrant & later comparing it with different novel synthetic superdisintegrant. By preparing a rapid disintegrating tablet of lamotrigine, rapid action of same can be achieved easily.
As we know that superdisintegrant plays the major role in rapid disintegration of tablet, thus it is very important to select a right or correct superdisintegrant in all respect which fulfill its purpose without effecting other parameters of tablet formulation and gives quicker and better result, when the number of formulation with different combinations of superdisintegrants (Natural & Synthetic) will be made then it would become very easy to evaluate and get the formulation with maximum desirable results.Lamotrigine being a poorly water soluble drug, needs modification to make them water soluble.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1347
INTRODUCTION :
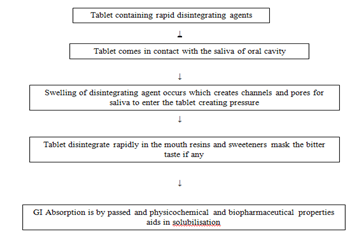
Rapid Disintegrating Tablets are solid dosage forms containing medicinal substances which disintegrate rapidly, usually in a matter of seconds, when placed on the tongue. Many pharmaceutical dosages are administered in the form of pills, granules, powders, and liquids. Generally, a pill design is for swallowing intact or chewing to deliver a precise dosage of medication to patients. The pills, which include tablets and capsules, are able to retain their shapes under Moderate pressure. However, some patients, particularly pediatric and geriatric patients, have difficulty swallowing or chewing solid dosage forms. Many pediatric and geriatric patients are unwilling to take these solid preparations due to a fear of choking. In order to assist these patients, several fast-dissolving drug delivery systems have been developed.
Fast-dissolving drug delivery in recent years, a variety of improved methods for delivering drugs has been developed with the aim of improving performance, convenience and compliance. Rapid Disintegrating Tablets disintegrate and or dissolve rapidly in the saliva without the need for water. Some tablets are designed to dissolve in saliva remarkably fast, within a few seconds, and are true fast-dissolving tablets. Others contain agents to enhance the rate of tablet disintegration in the oral cavity, and are more appropriately termed fast-disintegrating tablets, as they may take up to a minute to completely disintegrate. When put on tongue, this tablet disintegrates instantaneously, releasing the drug, which dissolves or disperses in the saliva. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablet dosage form. The advantage of orally disintegrating dosage Forms are increasingly being recognized in both industry and academia.
Rapid Disintegrating Tablets, as a novel dosage form, have several characteristics to distinguish them from the more traditional dosage forms. Taste-masking is of critical importance in the formulation of an acceptable RAPID DISINTEGRATING TABLETS. Traditional tablet formulations generally do not address the issue of taste masking, because it is assumed that the dosage form will not dissolve until passing the oral cavity.
[adsense:468x15:2204050025]
List of RAPID DISINTEGRATING TABLETS of LAMOTRIGINE Available in the Market
|
S.No. |
Brand Name |
API (in mgs) |
Manufactured by |
|
1 |
Lamepil |
Lamotrigine (25, 50, 100) |
Innova (Ipca) |
|
2 |
Lemetec |
Lamotrigine (5, 25, 50, 100) |
Protec (Cipla) |
|
3 |
Lamidus |
Lamotrigine (25, 50, 100) |
Zydus Neuro |
|
4 |
Lamitor-DT |
Lamotrigine (25, 50, 100) |
Mind(Torrent) |
|
5 |
Lyzin |
Lamotrigine (25, 50, 100) |
Pifer |
|
6 |
Lametec DT |
Lamotrigine (5, 25, 50, 100) |
Protec (Cipla) |
|
7 |
Lamidus Dispertab |
Lamotrigine (25, 50, 100) |
Zydus Neuro |
|
8 |
Lamictal |
Lamotrigine (5, 25, 50, 100) |
GSK |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SIGNIFICANCE OF RAPID DISINTEGRATING TABLETS
Ø Convenience of administration and accurate dose as compared to liquids.
Ø No need of water to swallow the dosage form, which is highly convenient feature for patients who are travelling and do not have immediate access to water.
Ø Ease of administration to patients who refuse to swallow a tablet, such as pediatric, geriatric, mentally ill, disabled and uncooperative patients.
Ø Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach; in such cases bioavailability of drugs is increased.
Ø Good mouth feel property of RAPID DISINTEGRATING TABLETSs helps to change the psychology of medication as “bitter pill” particularly in pediatric patients.
Ø Ability to provide advantages of liquid medication in the form of solid preparation.
Ø Rapid dissolution of drug and absorption, which may produce rapid onset of action.
MECHANISM OF TABLET DISINTEGRATION
The mechanism by which the tablets are broken into small pieces and then produce a homogeneous suspension is based on:
Ø Capillary action/ water wicking
Ø By swelling
Ø Air expansion /heat of wetting
Ø Due to disintegrating particle/particle repulsive forces
Ø Due to deformation
Ø Due to release of gases
Ø By enzymatic reaction
CHALLENGES IN THE FORMULATON OF ORALLY DISNTEGRATING TABLETS
a. Palatability
It is a formidable challenge for formulation scientists to mask the taste of bitter tasting drugs selected for Oral disintegrating tablets. As most drugs are unpalatable, orally disintegrating drug delivery systems usually contain the medicament in a taste-masked form. Hence, taste-masking of the drugs becomes critical to patient compliance.
b. Mechanical strength
In order to allow Rapid Disintegrating Tablets to disintegrate in the oral cavity, they are made of either very porous or soft molded matrices or compressed into tablets with very low compression force, which makes the tablets friable or brittle, and difficult to handle. Only few technologies can produce tablets that are sufficiently hard and durable to allow them to be packaged in multidose bottles.
c. Hygroscopicity / Moisture sensitivity
Several orally disintegrating dosage forms are hygroscopic and cannot maintain physical integrity under normal conditions of temperature and humidity. Hence, they need protection from humidity which calls for specialized product packaging.
d. Dose /Amount of drug
The application of technologies used for Rapid Disintegrating Tablets is limited by the amount of drug that can be incorporated into each unit dose. Molecules requiring high doses present mainly three challenges to the development of fast dissolve dosage forms; a) taste masking of the active ingredient, b) mouth feel or grittiness and c) tablet size. These challenges are not unrelated because most drugs will require taste masking, the amount of taste masking materials used in different dosage forms will depend on the drugs degree of bitterness relative to its dose, which will in turn affect the final tablet size.
e. Aqueous solubility
Water-soluble drugs pose various formulation challenges because they form eutectic mixtures, which result in freezing-point depression and the formation of a glassy solid that may collapse upon drying because of loss of supporting structure during the sublimation process. Such collapse sometimes can be prevented by using various matrix forming excipients such as mannitol than can induce crystallinity and hence, impart rigidity to the amorphous composite.
f. Size of tablet
The degree of ease in taking a tablet depends on its size. It has been reported that the easiest size of tablet to swallow is 7-8 mm while the easiest size to handle was one larger than 8 mm. Therefore, the tablet size that is both easy to take and easy to handle is difficult to achieve.
g. The Drug Property
Many drug properties could potentially affect the performance of fast dissolving tablets. For example, the solubility, crystal morphology, particle size and bulk density of a drug can affect the final tablet characteristics, such as tablet strength and disintegration.
h. Mouth feel
The RAPID DISINTEGRATING TABLETS should not disintegrate into larger particles in the oral cavity. The particles generated after disintegration of the RAPID DISINTEGRATING TABLETS should be as small as possible. RAPID DISINTEGRATING TABLETS should leave minimal or no residue in mouth after oral administration. Moreover addition of flavors and cooling agents like menthol improve the mouth feel.
i. Sensitivity to environmental conditions
RAPID DISINTEGRATING TABLETS’s generally should exhibit low sensitivity to environment conditions such as humidity and temperature as most of the materials used in an RAPID DISINTEGRATING TABLETS are meant to dissolve with minimum quantity of water.
ROLE OF SUPER-DISINTEGRANTS IN RAPID DISINTEGRATING TABLETS
Superdisintegrant plays the major role in Rapid disintegrating tablet. The disintegration efficiency is based on the force-equivalent concept (the combined measurement of swelling force development and amount of water absorption). Superdisintegrants are generally used at a low level in the solid dosage form, typically 1 – 10 % by weight relative to the total weight of the dosage unit. Common disintegrants used are Croscarmellose sodium (Vivasol, Ac-Di-Sol), Crospovidone (Polyplasdone), Carmellose (NS-300), Carmellose calcium (ECG-505), Sodium starch glycolate (SSG) etc. Recently few ion exchange resins (e.g. Indion 414) are found to have superdisintegrant property and are widely used in pharmaceutical industry. List of super disintegrants used for the formulation of orally disintegrating tablets with their mechanism of action were given in Table 2.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
List of Superdisintegrants for the Formulation of Rapid Disintegrating Tablets
|
Superdisintegrants |
Example |
Mechanism of action |
Special Comment |
|
Croscarmellose Ac-Di-Sol Nymce Zymce ZSX Primellose Solutab |
Cross linked cellulose |
Swells 4-8 folds in <10 seconds Swelling and wicking both |
Swells in two dimensions Direct compression or granulation Starch free |
|
Crosspovidone Crospovidone M Kollidon Polyplasdone |
Crosslinked PVP |
Swells very little and returns to original size after compression but act by capillary action |
Water insoluble and spongy in nature so get porous tablets |
|
Sodium Starch glycolate Explotab Primogel |
Crosslinked starch |
Swells 7-12 folds in <30 seconds |
Swells in three dimension and high level serve as sustain release matrix |
|
Alginic acidNFsatialgine |
Crosslinked alginic acid |
Rapid swelling in aqueous medium or wicking action |
Promote disintegration in both dry or wet granulation |
|
Soy polysaccharides Emcosoy |
Natural super disintegrant |
|
Does not contain any starch or sugar. Used in nutritional products |
TECHNOLOGIES FOR THE PREPARATION OF RAPID DISINTEGRATING TABLETS’s
1) Conventional Techniques
* Molding
* Freeze Drying
* Disintegrant addition
* Direct Compression
* Sublimation
* Mass extrusion
* Spray Drying
2) Patented technologies
* Flash tab technology
* Orasolv technology
* Wowtab technology
* Flashdose technology
* Zydis technology
* Durasolv technology
Delivery Mechanism Of Rapid DisintegratingTablet
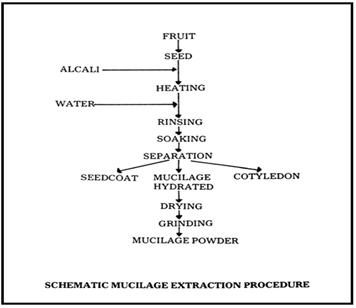
General Method Of Isolation Of Oscimum Basilicum Mucilage.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Conclusion:
The Rapid Disintegrating Tablets have potential advantages over conventional dosage forms, with their improved patient compliance; convenience, bioavailability and rapid onset of action had drawn the attention of many manufactures over a decade. The introduction of fast dissolving dosage forms has solved some of the problems encountered in administration of drugs to the pediatric and elderly patient, which constitutes a large proportion of the world's population. Hence, patient demand and the availability of various technologies have increased the market share of Fast dissolving tablets, which in turn prolongs the patent life of a drug. Keeping in view of the advantages of the delivery system, rapidly disintegrating dosage forms have been successfully commercialized, and because of increased patient demand, these dosage forms are expected to become more popular. Thus RAPID DISINTEGRATING TABLETS may be developed for most of the available drugs in near future.
Rapid Disintegrating Tablet is the selected drug delivery system as lamotrigine is used in the epileptic seizures thus it would be the best delivery system when one need a rapid onset of action.Choice of Lamotrigine which is BCS Class II drug, associated with its solubility problem thus onset of action is delayed which is not advisable for the patients with epileptic seizures. So by preparing a rapid disintegrating tablet of same would help in the formulation of such a dosage form which will overcome these mentioned disadvantages.Oscimum basilicum plant was procured from the local nursery and the seeds were separated from same.A comparative study of number of synthetic superdisintegrants was done and the two superdisintegrants were selected based on the objective of the project and feasibility which are Cross linked alginic acid and cross linked PVP.Other excipients which are important for the formulation of rapid disintegratimg tablet were selected.Isolation Of Oscimum Basilicum Seeds Mucilage From Oscimum Basilicum Seeds Was Performed Succesfully.Isolated Oscimum Basilicum Seeds Mucilage Was Characterized On The Basis Of Its Organoleptic Properties, Micromeritic Properties ( Angle Of Repose, Bulk Density, Tapped Density, Compressibility Index, Hausners Ratio, Particle Size) Along With Melting Point And Solubility Determination Was Also Performed.Physicochemical Characterization Of Oscimum Basilicum Seeds Mucilage Is Also Done. All the test results are allowing to proceed further to use it as a superdisintegrant. Preformulation studies on Lamotrigine were performed including identification of drug by organoleptic properties study, λ max determination, FTIR studies and other parameters including Melting Point Determination , pH Determination, Partition Coefficient Determination, Solubility studies (Quantitative and Qualitative), Standard Curve in different solvent. It would be possible to get the rapid onset of action of the anti epileptic drug Lamotrigine and thus can control the serious epileptic convulsions in the minimum time.
REFERENCES:
1. Adel M, Semreen M K, Qato M K. 2005. Fast dissolving dosage forms – technique. Pharm Tech. 2005; 25: 68-75.
2. Allen L V wang B. Method of making a rapidly dissolving tablet. US patent, 1986; 43: 123-131.
3. Dangagi P M, Sreenivas S A, Mastiholimath V S. Orodispersible tablets: New fangled drug delivery system a review. Ind. J Pharm. Edu. Res. 39:4.
4. Indurwade N H, Rajyaguru T H, Nakhat P D. Novel approach – Fast dissolving tablets, Indian Drugs. 2001; 39: 405-409.
5. Kaushik D. Mouth dissolving tablets: A review. Ind Drugs. 2004; 41: 187-193.
6. Kuchekar B S, Badhan A C. Design of fast dissolving tablets. Ind J. Pharm Edu. 2001; 35:150.
7. Slowson M, Slowson S. What to do when patients cannot swallow their medications. Pharm. Times. 1985; 51: 90-96.
8. Seager, H. Drug-deliver Products and the Zydis Fast-dissolving Dosage Form. J. Pharm. and Pharmacol. 1998; 50: 375-382.
9. Mizumoto T, Allen A, Loyd V. Methods for producing a rapidly dissolving dosage form. US patent 1996; 5: 576.
10. Reddy L H, Ghosh B, Rajneesh T. Fast dissolving drug delivery system a review of the literature. Ind J. of Pharm Sci. 2002; 64: 331-336.\
11. Ismail M , Ababio, G.; Habib, M. J., Central Properties and Chemical Composition of Ocimum Basilicum, 2006., 15 (1), 25-45
12. Baritaux O, Gangwar S, Singh S, Garg G, Pawar V, Sharma P K Effects of drying and storage on herbs of Ocimum basilicum L., 1992; 2:1-7.
13. Akgul A, Alanazi F K, Ibrahim M, Bagory E, Ibrahim A A, Mohsen A B, Moustafa A Analysis of composition of sweet basil (Ocimum basilicum L.) cultivating in Turkey, 1989; 16: 117-21
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









