About Authors
1AGARWAL POONAM*, 2RATHORE KAMAL SINGH, VIJAY KAVITA2
1Department of Pharmaceutics, Bhupal Nobles’ College of Pharmacy Udaipur, Rajasthan,
2Faculty, Jaipur, College of Pharmacy, Jaipur India, 313001.
Email : charmiagarwal823@gmail.com
ABSTRACT
The Covid-19 pandemic has brought detrimental impact for the human and economic costs, majorly due to the lack of specific treatment. At this stage, drug repurposing is the better option to arrive at treatments of covid-19 in short-term instead of complementary to immunotherapy. Currently, most of the examples of drug repurposing can be seen that are undertaken in clinical trials for the potential Covid-19 treatments. Food Drug Administration (FDA) approved a repurposed antiviral drug i.e. remdesivir in October 2019, as the first treatment of covid-19. It will cover different prospects of repurposing which are undergoing non-clinical and clinical trials or with some level of evidence that are emerging from clinical studies are overviewed.In this review article will provide deep understanding about several emergence of drugs which are going to repurposed for dealing with covid-19 situation. We will discuss about real case studies whereas existing drugs are using to treat patients with covid-19 by executing drug repurposing strategy.In last, we will highlight some commercial and intellectual barriers to drug repurposing and strategies in order to facilitate equitable access for incoming therapeutic solution.
INTRODUCTION
The new and highly contagious severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) is the major reason of outbreak which triggered novel coronavirus disease, covid-19 as resulted it represents a pandemic threat to worldwide health. More than 65 million confirmed cases along with 1.5 million deaths worldwide received yet that results in economic losses and unprecedented human (1,2). Currently, there is no treatment option available which can help to get rid-off from the deadly contagious disease, Covid-19. However, scientific communities and international politics have already retorteddynamically to offer new therapeutic and diagnostic solutions. Regulatory agencies across globe have executed fast-track process to acceleratethe development and marketing authorization of the diagnostic and therapeutic solutions against coronavirus disease. For example, The US-FDA has introduced a special emergency program to advance the development of coronavirus therapies as the Coronavirus Treatment Acceleration Program (CTAP) (3). This website (CTAP) lists more than 560 drug development programs in the planning stage, above 370 trials have reviewed by the agency. There are five covid-19 treatments that are authorized for the emergency use (encompassing, very recently, one treatment (remdesivir) and anti-arthritis drug barcitinib, have approved by FDA against Covid-19. Though, there are various international collaborativedrug development initiatives arose to give instant responses to the current crisisi.e. crowd sourcing, private-public association, open innovation among other models (4), (5). Our experts may highlight small molecule and immunotherapies, encompassing hyperimmune globulin, monoclonal antibodies and convalescentplasma transfusion among emerging treatments against Covid-19 (6), (7).In fact, significant focus has placed on the therapeutic interventions that have provided a solution such as vaccines in the short-mid-term.It is not quite shocking that so far only approved drug therapy. Most of the researchers are undergoing or underwent clinical trials for the repurposed drug.
SIGNIFICANCE OF DRUG REPURPOSING
Drug purposing is considered emerging strategy wherein existing or available drugs, already have been done clinical trials and tested safe in humans, are re-organized against difficult diseases.To implement drug repurposing strategy, there is a reason such as, we all know very well, outbreak of covid-19/20has brought worst result economic loss, poor global public health. However, all efforts must be made towards prevention of 2019/2020 outbreak.Average cost of the de novo development stated around USD 1 billion USD. To combat with sever acute respiratory syndrome coronavirus 2 (SARS-CoV-2) takes a snapshot look towards drug repurposing strategy which is also known as drug repositioning or reprofiling (8). These strategy promises to determine antiviral agents for the Covid-19 disease in a critical time. Remdesivir has received as conditional marketing authorization from the European Commission because they investigated that this drug contains antiviral molecule (9). This drug is effective for the treatment of covid-19 in adults and teenagerswith pneumonia who needs supplemental oxygen. Even, no other treatments have been approved to date.In past few years, there are lots of pharmaceutical companies which are developing new drugs with the discovery of novel biological targets by implementing repositioning strategy in the drug discovery and development program(8), (10). This strategy is considered highly efficient, low-cost, time-saving and minimum risk of failure.
To find new molecular entities (NME) through traditional approach or de-nova drug discovery process is quite lengthy, expensive and time consuming. Drug repurposing is a lot like recycling and has become one of the cheaper and most fasted way to bring treatment in the market, especially for those who survives with rare and ultra-rare diseases. This drug repurposing strategy has been gained substantial momentum lately, around one out of three approvals links to drug repurposing in recent years.While repurposed therapeutics currently generates around 25% revenue of the pharmaceutical industry(11). So, most of the pharmaceutical companies has been progressively integrated this approach into the life cycle management of the pharmaceutical products (11) (12). Previous knowledge on the proposed repurposed researchers, such as manufacturing data and pharmacokinetic assists shorten the long drug development timeline. It diminishes cost of the development as well. The potential advantage of the drug repurposing is to prove safety of the repurposing researchers, which are less likely to fail at initial clinical trials or may bypass Phase 1 trials if the drug compatibility with the original is met (12), (13)).
World Health Organization proposed a document on Draft Target Product Profiles (TPP) that is based on the treatment of Covid-19. With this proposed document, organization highlightspreferred profiles which are majorly to be instantly fulfilled through repurposed drugs(14), for instance:
1. Small children and pregnant women are involved in the target population.
2. Safety profile superior or similar to existing therapeutic agents.
3. Absence of adverse events or detrimental events that needs to be monitoring.
4. Efficiency to rapid scaling up at different costs per dose that enable broad use.
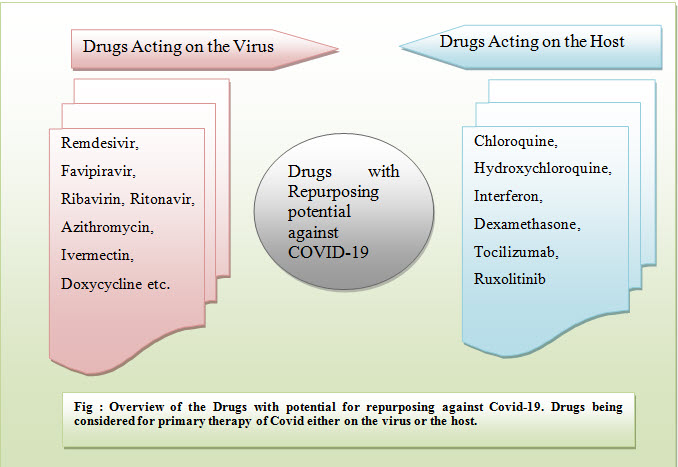
There are number of things that considered relative advantageous of drug repurposing (comparison with de novo drug discovery). Currently, so many ongoing covid-19 project as well as clinical trials emphasizes on repurposed matter (15). The reason behind is that it is essential to address urgent therapeutic needs for the orphan diseases in a cost and time efficient manner. Researchers are putting more efforts on discriminating virus-focused treatments from host-based treatment.
Virus and Host Focused drug repurposing (13, 15)
Immunoprophylaxis (vaccines) can be defined as the priming for activation of host immune response against particular pathogens. It is considered one of the safest approaches among others to control or eradicate various virus diseases (i.e. influenza virus, hepatitis virus, measles, papillomavirus). In the early 80s’HIV was considered global pandemic, caused by the human immunodeficiency virus, which advanced modern antiviral drug development. To date, the development of antiviral therapies has become centred on chronic viral diseases i.e. Hepatitis C virus (HCV) and HIV, which are the major reason for 68% of the FDA antiviral drug approvals (16). In contrast, only fewer drugs come in market authorization to treat sever acute respiratory diseases. Currently repositioning strategy is majorly focused against SARS-CoV-2 in exploring the cross-reactivityof biological and chemical entities, with the particular immunomodulating agents(16), (17). For example, first actions, involving clinical trials, were proposed to test the therapeutic efficacy of antiviral drugs targeting viral RNA replication (i.e. Remdesivir) (20) and protease (i.e. ritonavir, lopinavir (17)). While adjunctive treatment is completely based on monoclonal antibodies (i.e. tocilizumab (21) or drugs (i.e. dexamethasone (27), chloroquine (22)), have aimed to diminish inflammatory responses which already have been assayed in covid-19 patients. Ivermectin is also one of the great examplesof a host-directed agent that explored for its potential in order to hampering SARS-CoV-2 replication. FDA also has approved macrocyclic lactone drug for the treatment of several parasitic diseases,because this drug is belonging from broad spectrum category and its antiviral activity has been linked with the resistance of the host nuclear transport importin. However, this is hijacked by the viruses by suppressing the antiviral responses or to translocate viral protein which are essential for replication. The pharmacological dose of Ivermectin (23)may impart anti-SARS-CoV-2 activity. Even many of drugs, alone or in combination, were or currently subjected of clinical evaluation. Globe’s largest trials of covid-19 therapies have been executed among existing drugs remdesivir, lopinavir/ritonavir, hydroxychloroquine combination but results were highly disappointed. Last October, FDA permittedIV remdesivir for the treatment of covid-19 in adults and adolescent from 12 years of age who requires supplemental oxygen.
Recent surveying study analysing IFNa in Covid-19 patients linkedearly administration of the cytokine along with decreased mortality and its late use along with high mortality or delayed recovery (24). Thus, there are some other examples, based on them researchers are focusing on drug repositioning research against Covid-19 Wirth novel and steady finding on the pathophysiology of SARS-CoV-2 infection (26).
New intervention in drug repurposing strategy
Currently, most of the researchers are doing study on existing drugs so that they can repurposed drug to combat with current pandemic. Recently, a scientist Achal Agarwal and HOD & CEO, NK Agarwal of Memorial Chemistry Research Lab, Macsen Drugs, Udaipur have quoted that Methylene Blue (a 120-year-old drugs), is repurposed for Covid-19 treatment.
Methylene Blue
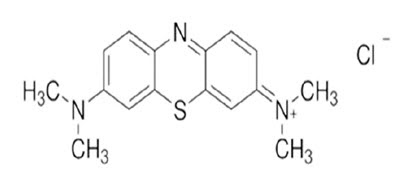
• IUPAC Name: 3,7 bis (dimethylamino)-phenothiazin-5-ium chloride
• Molecular Formula: C16H18N3SCl
• Molecular Weight: 319.86 g/mol
• This is also known as MethylthionineChloride. It is a Planar Tricyclic heteroaromatic diamino phenothiazine compound.
• It was firstly synthesized by Heinrich Caro in 1876 and started to be used as an antimalarial from since 1800s. Methylene Blue is considered as the Globe’s first Synthetic Drug.
• It is used as an antimalarial at the end of World War II.
• Antiseptic properties is are also posses’ by methylene Blue.
• In 1930s, it was started to being used for the treatment of Methemoglobinemia which is still worldwide approved to deal with Methemoglobinemia.
• However, methylene blue is given in combination to inactivate the viral load (inactivate broad spectrum of viruses encompassing HIV viruses, Hepatitis viruses etc.) in blood plasma before transfusion.
Pharmacological Properties of Methylene Blue :
• Methylene Blue contains a broad-spectrumAntiviral Action with or without presence of light by different mechanismsi.e. Interaction b/w the RNA pairs, Protein binding, Singlet Oxygen generation etc.
• Methylene Blue decreases oxidative stress and scavenges Reactive oxygen species as well as oxidative free radicalscaused by its antioxidant property.
Methylene Blue as an available drug for various indications, worldwide :
• Methylene Blue injection approved worldwide as first-in-line treatment of Methemoglobinemia.
• Methylene Blue Injection has achieved a ‘Grandfathered Drug’ profile in USA as a pre-1938 drug. Methylene Blue has long history of safety and use so it is titled as a ‘Grandfathered Drugs’ which do not require any type of FDA approvals in United State.
• This drug is available in different forms and using in number of countries till now i.e. URIBEL & UROGESIC BLUE Capsules (contains 10mg Methylene Blue) in US as Urinary antiseptic,DOMITAZOL (OTC Drug, contains 25mg Methylene Blue) in Vietnam as Urinary Septic, Methylene Blue MMX tablets (contains 200mg of Methylene Blue)approved in Europe in 2020 August for visualization of colorectal lesion while Colonscopies, UROLENE BLUE (65mg Methylene Blue tablets) is another effective Grandfathered Drugs in US to diagnose urinary septic, CYSTEX as OTC drug (contains 20mg Methylene Blue USP and other ingredients) in Brazil as urinary antiseptic.
• Methylene Blue IV is pretty known for the treatment of Cytokinin Release Syndrome, Vasoplegia Syndrome etc.
Known Safe Dosages of Methylene Blue in numerous routes of Administration and Indications :
• Methylene Blue can be given by Intravenous route as Methemoglobinemia. The safe dosage of this drug can be given up to 2 mg/ Kg in short single dose is considered safest. This information is collected by USFDA Medical Review of Provayblue NDA: 204630 (29).
• It can be administered orally as Colonoscopy. The safest dosage is up to 400mg (single dose) that data gathered from an article: Methylene blue MMX® tablets for chromo endoscopy. DOI: 10.1016/j.cct.2011.11.006 (30).
• It is also administered orally as antimalarial drug. The safest single dose of this drug is about 500mg. This statement is quoted by Ingeborg Walter-Sack, et.al, DOI: 10.1007/s00228-008-0563-x (31).
Contraindication of Methylene Blue
• Methylene Blue never administered to patients who having history of G6PD (Glucose 6 Phosphate) deficiency. The reason behind is that it can develop haemolysis in G6PD patients.
• Pregnant women and lactating women should not be administered this drug.
Discussion and Result
Antiviral Activity of Methylene Blue in Covid-19 infection
Invitro Study 1
“Methylene Blue has capability to inhibit the SARS-CoV-2 Spike-ACE2 Protein-Protein interaction. It is a mechanism of action of Methylene Blue that can play prime role in its Antiviral Activity against Covid-19”.This claim is done by experts Damir Bojadzic, Oscar Alcazar, Peter Buchwald in Diabetes Research Institute, University of Miami, FL, United States (27).
Key findings of invitro study:
• Methylene Blue represented a low micromolar half maximal inhibitory concentration (IC50) around 3μM in the Assay of ELISA-based Protein Binding.
• While in an assay of SARS-CoV-2 spikes pseudoviral entry into ACE2 imparting HEK293T cells, Methylene Blue showed a low micromolar half maximal inhibitory concentration (IC50) around 3.5μM in the form of a concentration dependent manner (32).
Conclusion
Based on the above discussion can be concluded that Methylene Blue can effectively inhibit protein-2 interaction b/w SARS-CoV-2 Spike protein at low micromolar concentration. It can inhibit viral entry of SARS-CoV-2 spikes bearing pseudovirus into ACE2 expressing cells by normal clinical dosage around 200 mg (TID, Oral).
Invitro Study 2 (32)
“Methylene Blue resits the replication of SARS-CoV-2 in vitro” statement illustrated by Mathieu Gendrot, Julien Andreani, Isabelle Duflot et.al 2020 in International Journals of Antimicrobial Agents (2020).
Key Findings:
• Methylene Blue has capability to inhibit replication of SARS-CoV-2.
• It shows cytotoxic concentration (CC50) of > 100 μM in Vero E6 cells.
• Invitro antiviral activity of Methylene Blue (0.75 μM) was found more higher as compared other drugs i.e. azithromycin (20.1 μM), remdesivir (23 μM), ritonavir (>100 μM) or lopinavir (26.6 μM).
Conclusion
Based on the above discussion it is summarized that methylene blue can inhibit (90% inhibition) the SARS-CoV-2 virus. Antiviral activity of Methylene Blue is higher as compared other currently used drugs.
Proposed Route of Administration of Methylene Blue in Covid-19 patients (33) :
Methylene Blue administers intravenously in mild to moderate Covid-19 patients which demonstrated good response in controlling the infection.This drug in severe Covid-19 patients have also shown reduced morality rates.
Methylene Blue Inj. 1% USP is existing in Ampules of 10 mL (10 mg/ml, 100 mg in one ampule).
Following process is followed for the administration:
• Administer 100 mg to an adult patient by IV in 20-30 minutes.
• Later 4-5 hours give an IV infusion of 15-18 mg per hour.
• Administer drug total 200 mg within 24 hours.
• Repeat this drug for three days.
Currently, Phase1, Phase IIand Phase III clinical trials of methylene blue have been done in covid-19 patients and achieved results are desirable. Recently, Dr. Rajesh Dere, who is the in-charge of BKC Jumbo COVID centre, Mumbai has gained breakthrough results in treating serious Covid-19 patients by using 120-year-olddrug Methylene Blue Injection. Around 120 patients with severe Covid-19 were admitted in ICU who were administered the drug Methylene Blue intravenously. Administration of this drug has brought desirable results in the BKC Covid Centre, Mumbai.
CONCLUSION
This article has been concluded about significance of repurposing drug and causes for executing this strategy.In this article it has summarized that how experts at different centres are putting efforts to make existing drugs in to repurposed manner. Virus and host focused drug repurposing term has also explained in it which stated that how available drugs alone or with combination are using to deal with globally covid-19 pandemic. There are number of drugs i.e. remdesivir, dexamethasone, chloroquine, hydro-chloroquine, combination of interferon and cytokinin, ivermectin etc. used to treat severe Covid-19/20 pandemic in which remdesivir was approved by number of regulatory authorities to treat covid-19 patients. However, above mentioned drugs were used in the form of repurposed drug. Methylene Blue is the new intervention of repurposed drug which is evident by experts to have potential to treat severe patient with Covid-19 because it has much antiviral action rather than other drugs like remdesivir. Invitro case studies first and second, finding and their conclusion has been concluded in this article. Pharmacological actions, route of administration and existing applications have been concluded in it. With the support of invitro study 1 and invitro study 2, it has illustrated that Methylene Blue is a Repurposable Drugs against SARS-CoV-2. The Viral inhibition of this drug is much better than Remdesivir in both type SARS-CoV-2 strains. Drug repurposing strategy is proving quite time and cost-effective in this peak period.
REFERENCES
1. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. [Accessed on October 10th 2021]. Available from: https://covid19.who.int/
2. Nicola M, Alsafi Z, Sohrabi C, et al. (2020); the socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg.; 78:185–193.
3. Food and Drug Administration. Coronavirus Treatment Acceleration Program (CTAP). [Accessed on October 10th 2021]. Available from: https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap.
4. Chodera J, Lee AA, London N, et al. (2020);Crowdsourcing drug discovery for pandemics. Nat Chem. 12:581.
5. Chesbrough H. (2020); to recover faster from Covid-19, open up: managerial implications from an open innovation perspective. Ind Mark Manag. 88:410–413.
6. Islam MT, Nasiruddin M, Khan IN, et al. Perspective on emerging therapeutic interventions for COVID-19. Front Public Health. 2020; 8:281.
7. Sarkar C, Mondal M, Islam MT, (2020); Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front Pharmacol. 11. DOI:10.3389/fphar.2020.572870.
8. Ashburn TT, Thor KB (2004); Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3: 673-683.
9. Cao, Y. C., Deng, Q. X., & Dai, S. X. (2020); Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Medicine and Infectious Disease. 101647. https://doi.org/10.1016/j.tmaid.2020.101647.
10. Cha, Y., Erez, T., Reynolds, I.J., Kumar, D., Ross, J., Koytiger, G., Kusko, R., Zeskind, B., Risso, S., Kagan, E., Papapetropoulos, S., Grossman, I., Laifenfeld, D. (2018); Drug repurposing from the perspective of pharmaceutical companies. British Journal of Pharmacology, 175(2), 168–180. https://doi.org/10.1111/bph.13798.
11. Naylor S, Kauppi DM, Schonfeld JP (2015); Therapeutic drug repurposing, repositioning and rescue part II: business review. Drug Discovery World. 16(2):57–72.
12. Naylor S, Kauppi DM, Schonfeld JP (2015); Therapeutic drug repurposing, repositioning and rescue: part III: market exclusivity using intellectual property and regulatory pathways. Drug Discovery World. 16(3):62–69.
13. Oprea TI, Overington JP (2015); Computational and practical aspects of drug repositioning. Assay Drug Dev Technol. 13(6):299–306.
14. World Health Organization. WHO target product profiles for COVID-19 therapeutics. (2020); [cited October 10th 2020]. Available from: https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-therapeutics.
15. Chaudhuri S, Symons JA, Deval J.(2018); Innovation and trends in the development and approval of antiviral medicines: 1987-2017 and beyond. Antiviral Res. l; 155:76–88.
16. Forum of International Respiratory Societies (2017); The global impact of respiratory disease. Second ed. Sheffield: European Respiratory Society.
17. Hung IF, Lung KC, Tso EY, et al.(2020); Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 395:1695–1704.
18. Pushpakom S, Iorio F, Eyers PA, (2019); Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 18:41– 58.
19. Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020); Remdesivir for the treatment of covid-19 — preliminary report. N. Engl. J. Med. [Epub ahead of print].
20. Campochiaro, C., Della-Torre, E., Cavalli, G., De Luca, G., Ripa, M., Boffini, N., et al. (2020); Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 76, 43–49.
21. Maskin LP, Olarte GL, Jr PF, (2020); High dose dexamethasone treatment for Acute respiratory distress syndrome secondary to COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 21:743.
22. Cha, Y., Erez, T., Reynolds, I. J., Kumar, D., Ross, J., Koytiger, G. Laifenfeld, D. (2017); Drug repurposing from the perspective of pharmaceutical companies. British Journal of Pharmacology, 175(2), 168–180.
23. Caly L, Druce JD, Catton MG, (2020); The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 3:104787.
24. Wang N, Zhan Y, Zhu L, (2020); Retrospective multicentre cohort study shows early interferon therapy is associated with favourable clinical responses in COVID-19 patients. Cell Host Microbe. 28:455–464.
25. Singh TU, Parida S, Lingaraju MC, (2020); Drug repurposing approach to fight COVID-19. Pharmacol Rep. 72:1479–1508.
26. Gautret P, Lagier JC, Parola P, (2020); Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 56:105949.
27. Talevi A, Bellera CL (2020); Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin Drug Discov. 15:397–401.
28. USFDA Medical Review of Provayblue NDA: 204630, 2016. (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/204630Orig1_toc.cfm).
29. Repici, A., Di Stefano, A. F. D., Radicioni, M. M., Jas, V., Moro, L., & Danese, S. (2012); Methylene blue MMX® tablets for chromoendoscopy. Safety tolerability and bioavailability in healthy volunteers. Contemporary Clinical Trials, 33(2), 260–267.
30. Walter-Sack, I., Rengelshausen, J., Oberwittler, H., Burhenne, J., Mueller, O., Meissner, P., & Mikus, G. (2008); High absolute bioavailability of methylene blue given as an aqueous oral formulation. European Journal of Clinical Pharmacology, 65(2), 179–189.
31. Mathieu Gendrot, Julien Andreani,(2020); Methylene blue inhibits the replication of SARS-Cov-2 in vitro, International Journal of Antimicrobial Agents.
32. Bojadzic D, Alcazar O, Buchwald P. Methylene Blue inhibitsinvitro the SARS-CoV-2 Spike – ACE2 Protein-Protein Interaction a Mechanism That Can Contribute to Its Antiviral Activity against COVID-19. bioRxiv [Preprint] 2020; [cited 2020 October 21]. Available from https://doi.org/10.1101/2020.08.29.273441.Available at [https://www.frontiersin.org/articles/10.3389/fphar.2020.600372/full].









